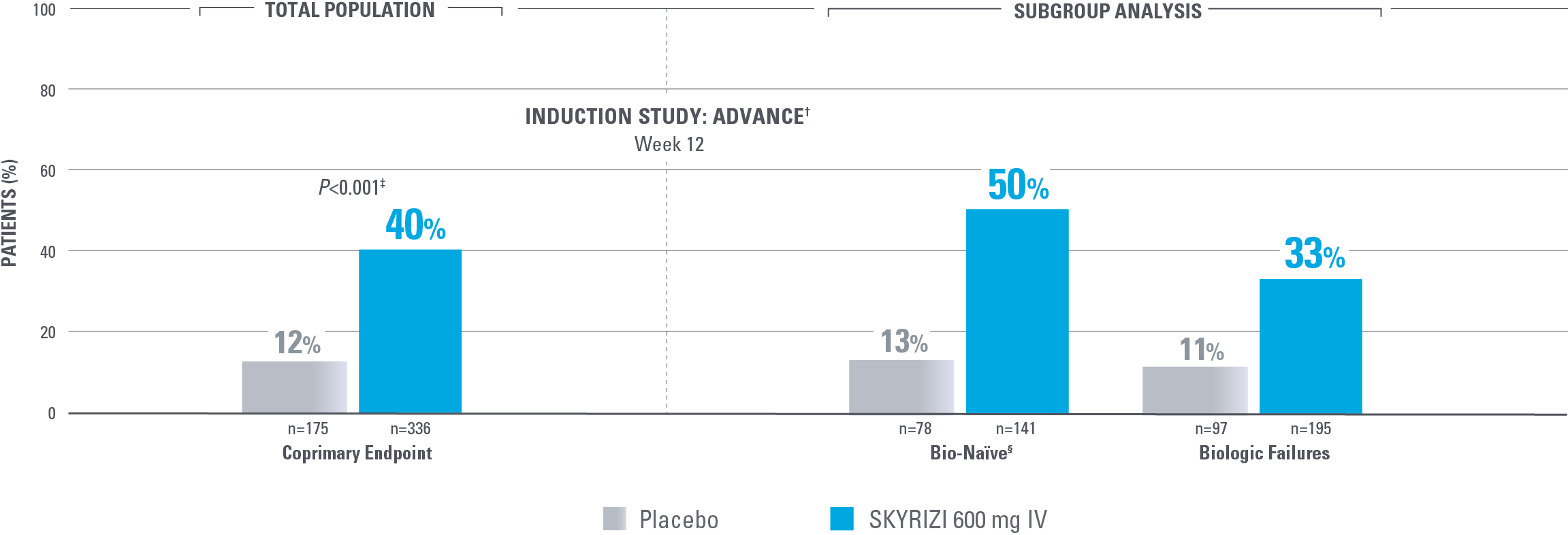
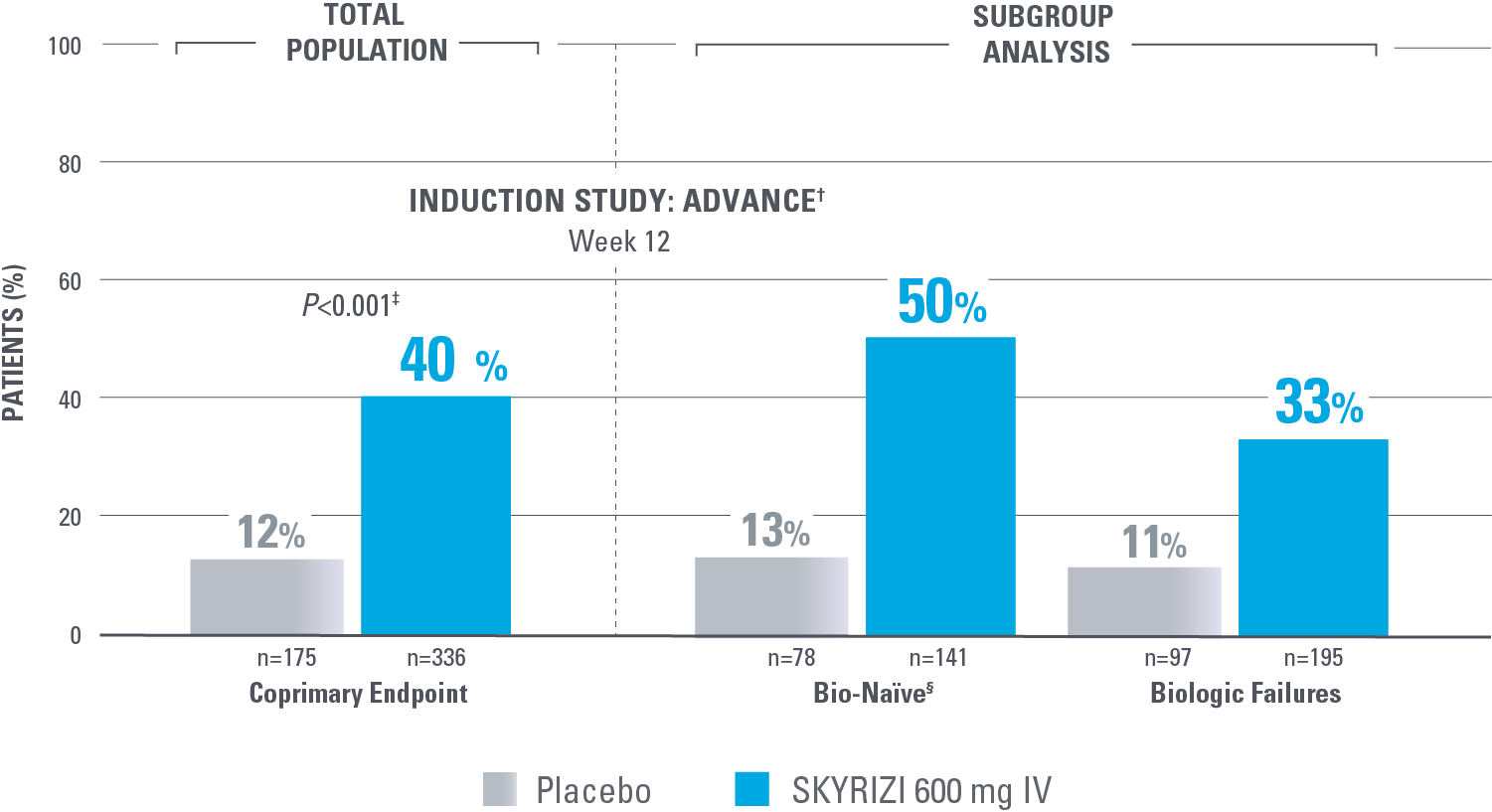
ENDOSCOPIC RESPONSE AT WEEK 12: A STEP TOWARD HEALING THE MUCOSA
Endoscopic Response in Bio-Naïve and Biologic Failure Patients1*
PRIMARY ENDPOINTS IN SEQUENCE
*NRI-C: Nonresponder imputation COVID-19, incorporating multiple imputation for missing data due to COVID-19 infection or logistical restrictions used for categorical endpoints; patients with missing data for all other reasons were counted as nonresponders.2
†The total population in the MOTIVATE and ADVANCE studies includes patients dosed with risankizumab 1200 mg IV, which is not an approved dose for CD.1
‡Statistically significant under multiplicity control for SKYRIZI vs placebo comparison (P<0.001).
Endoscopic response: Decrease in SES-CD >50% from baseline (or a decrease of at least 2 points for subjects with a baseline score of 4 and isolated ileaI disease), as scored by central reviewer.
§Among the patients without prior biologic failure, 87% were naïve to biologic therapy and the remaining 13% had received a biologic but never failed or demonstrated intolerance.1
MOTIVATE was also an induction study that included all biologic failure patients (in the total population, 11% of placebo patients and 29% of SKYRIZI patients achieved endoscopic response).
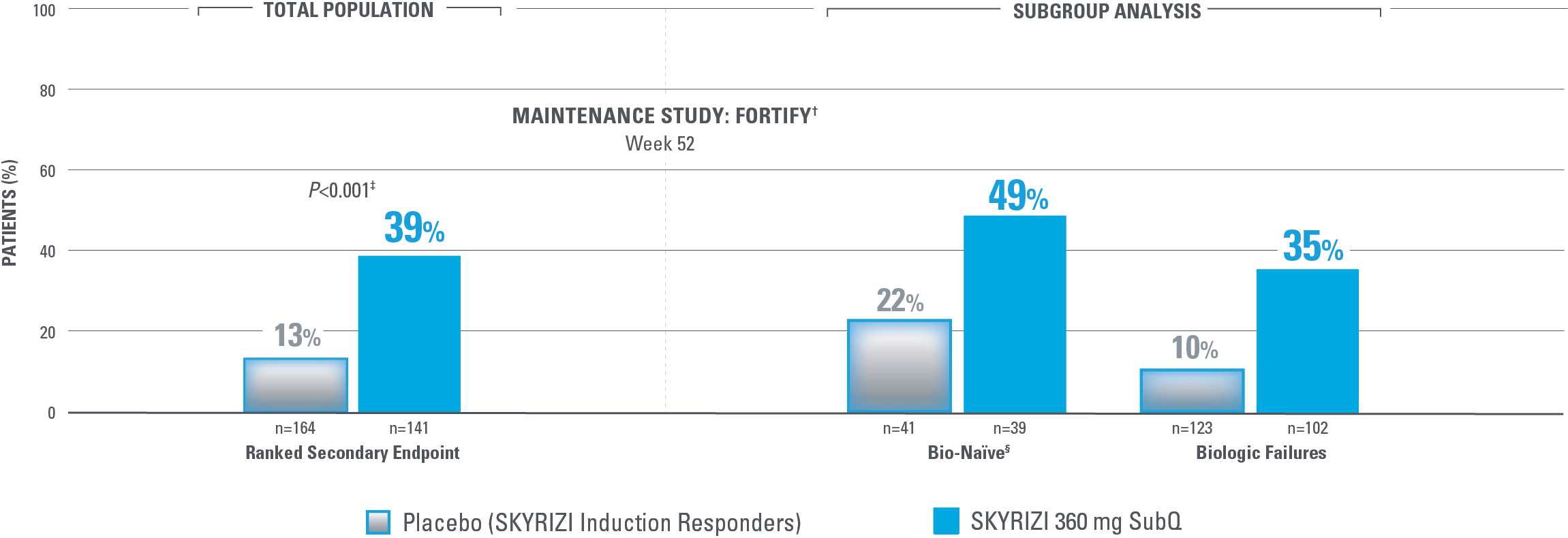
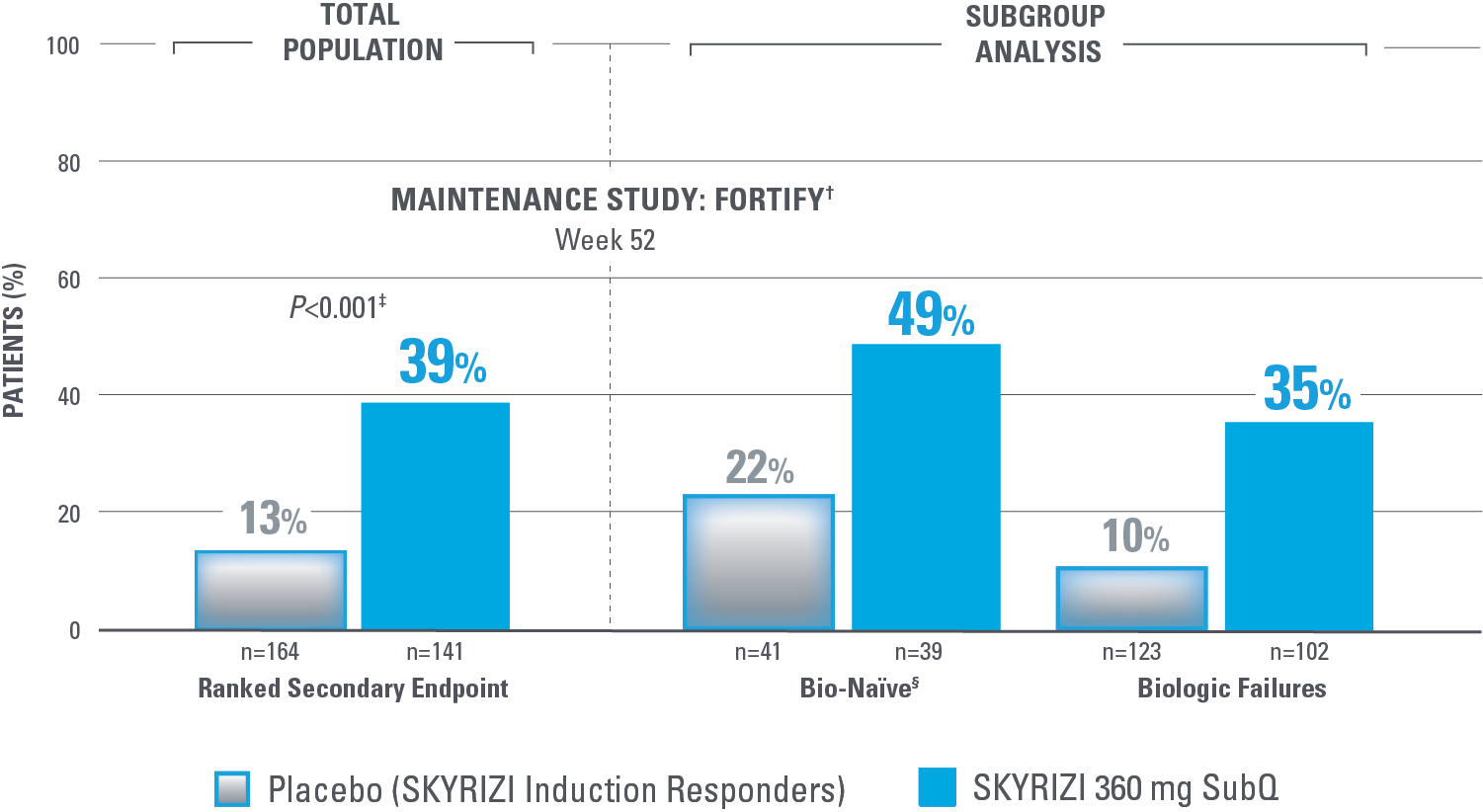
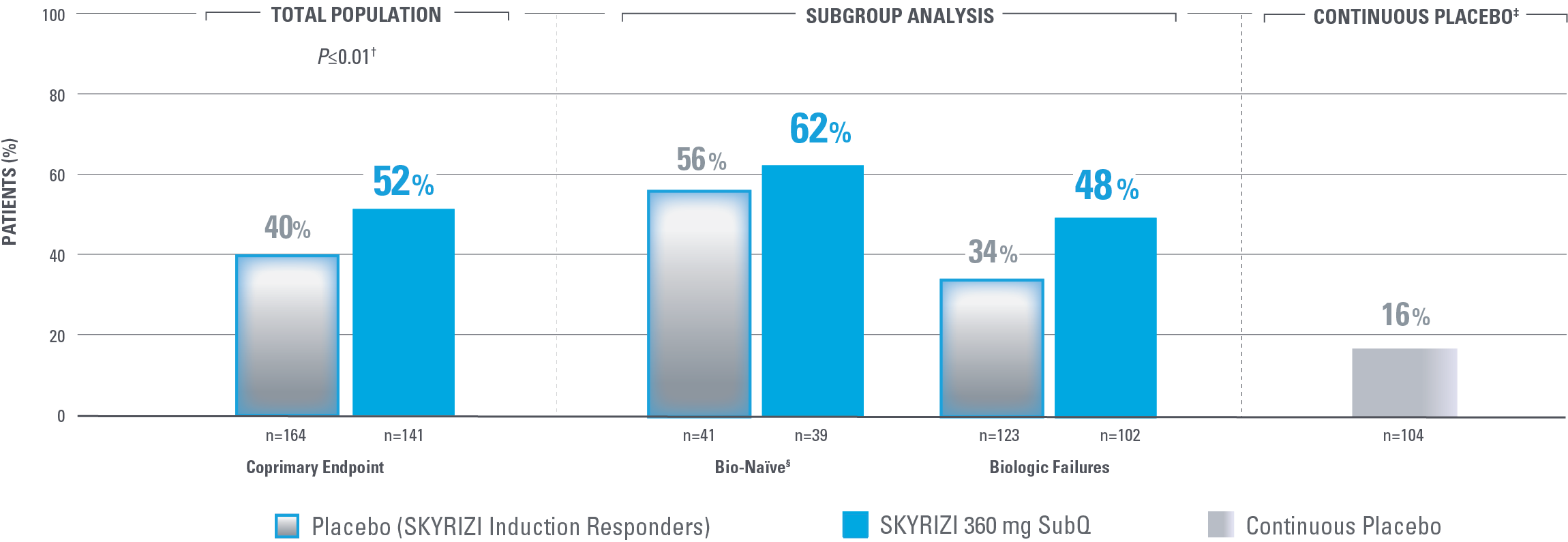
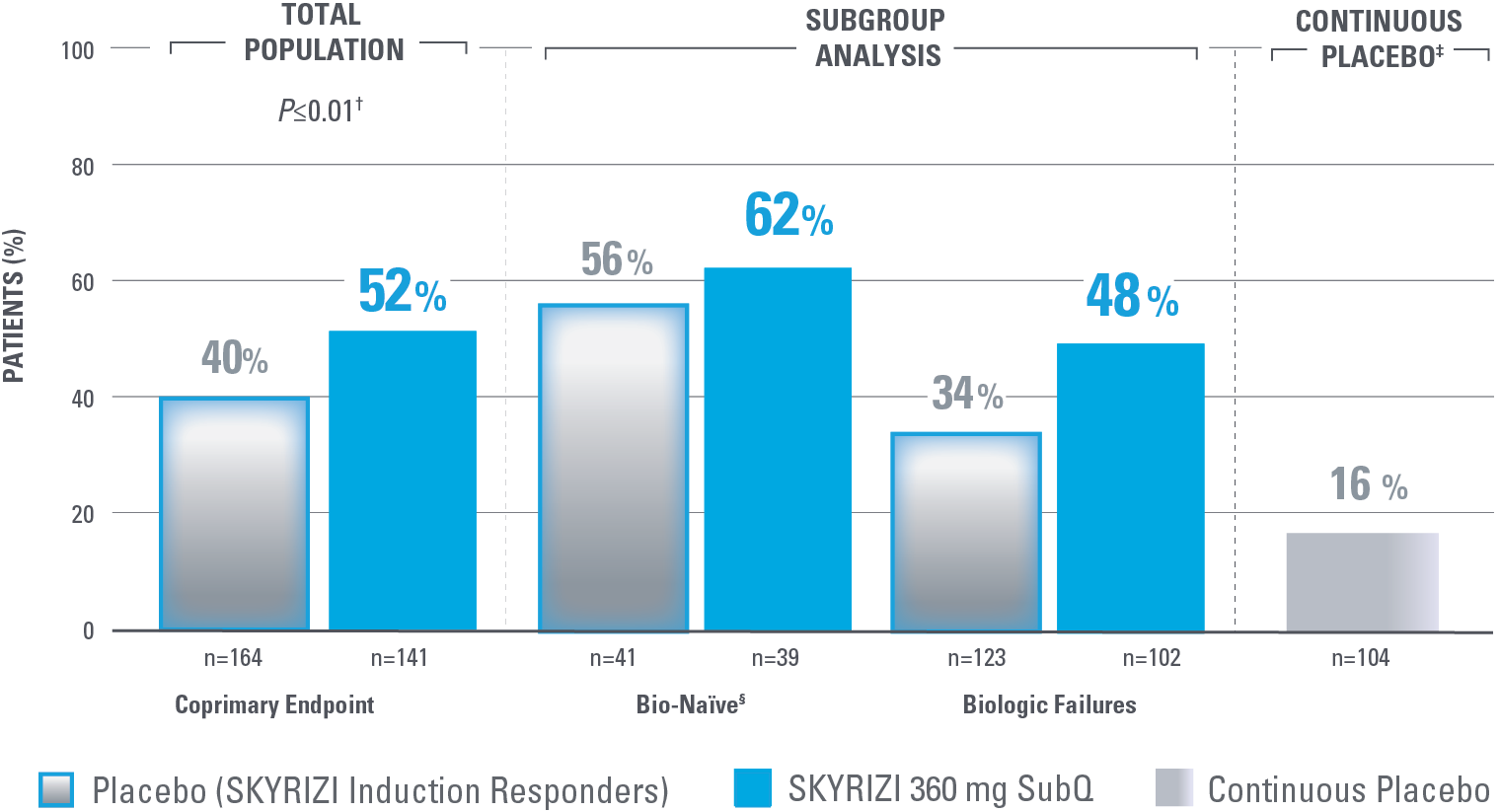
ENDOSCOPIC REMISSION AT WEEK 52
Endoscopic Remission in Bio-Naïve and Biologic Failure Patients1,3*
*NRI-C: Nonresponder imputation COVID-19, incorporating multiple imputation for missing data due to COVID-19 infection or logistical restrictions used for categorical endpoints; patients with missing data for all other reasons were counted as nonresponders.3
†The total population in the FORTIFY study includes patients dosed with risankizumab 1200 mg IV induction and risankizumab 180 mg SubQ maintenance, which are not approved doses for Crohn’s disease.1
‡Nominal P<0.001 SKYRIZI vs placebo comparison without overall type I error control. Nominal P values denote data not controlled for multiplicity. No clinical inferences can be drawn.1
Endoscopic remission: SES-CD ≤4 and at least a 2-point reduction vs baseline and no subscore >1 in any individual variable, as scored by a central reviewer.
§Among the patients without prior biologic failure, at FORTIFY Week 0, 93% were naïve to biologic therapy and the remaining 7% had received a biologic but never failed or demonstrated intolerance.4
NOMINAL SIGNIFICANCE: Since the coprimary endpoint of clinical remission in the 180 mg randomized arm did not reach statistical significance, the secondary endpoints were not statistically significant per the statistical testing plan. Therefore, the P value for this ranked secondary endpoint is considered nominally significant.
SIGNIFICANT CLINICAL REMISSION AT WEEK 52 IN THE TOTAL POPULATION
Clinical Remission in Bio-Naïve and Biologic Failure Patients1,5*
*NRI-C.3
†Statistically significant under multiplicity control for SKYRIZI vs placebo comparison (P≤0.01).1
‡NRI-NC: Nonresponder imputation no COVID-19; missing values were imputed as nonresponse with no special data handling methods for data missing due to COVID-19.3
§Among the patients without prior biologic failure, at FORTIFY Week 0, 93% were naïve to biologic therapy and the remaining 7% had received a biologic but never failed or demonstrated intolerance.4
Continuous placebo data are not intended for direct comparison.
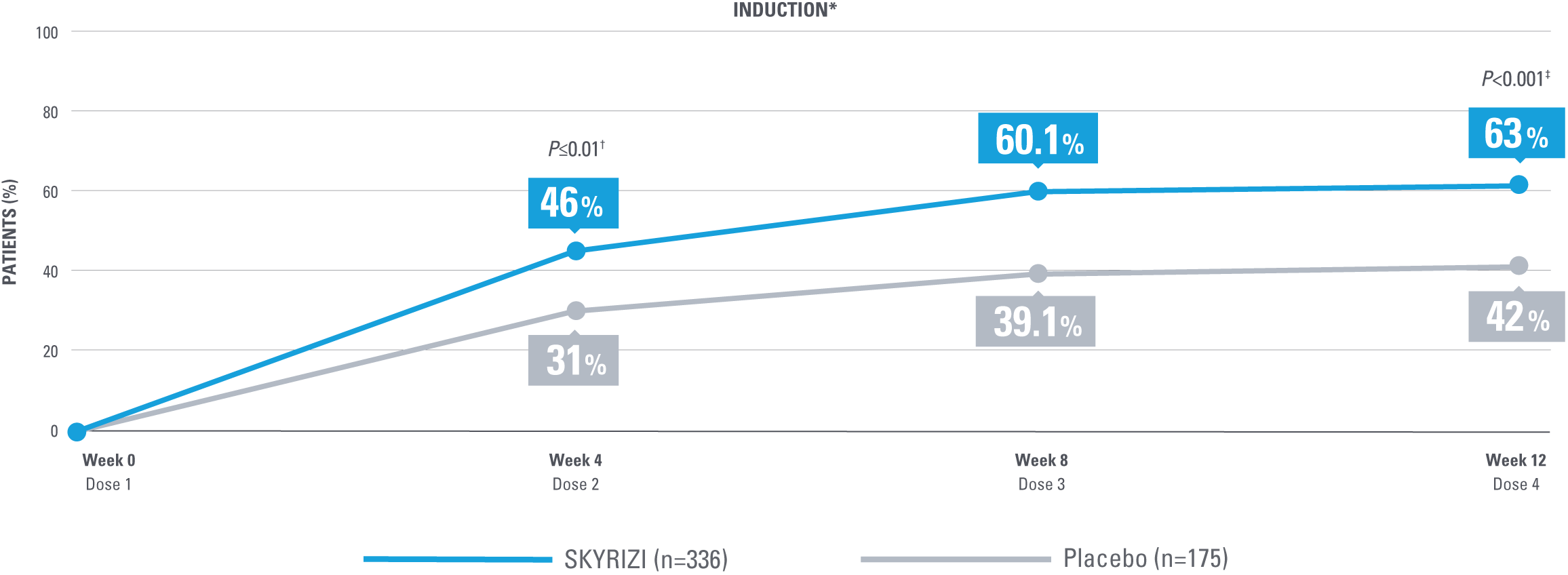
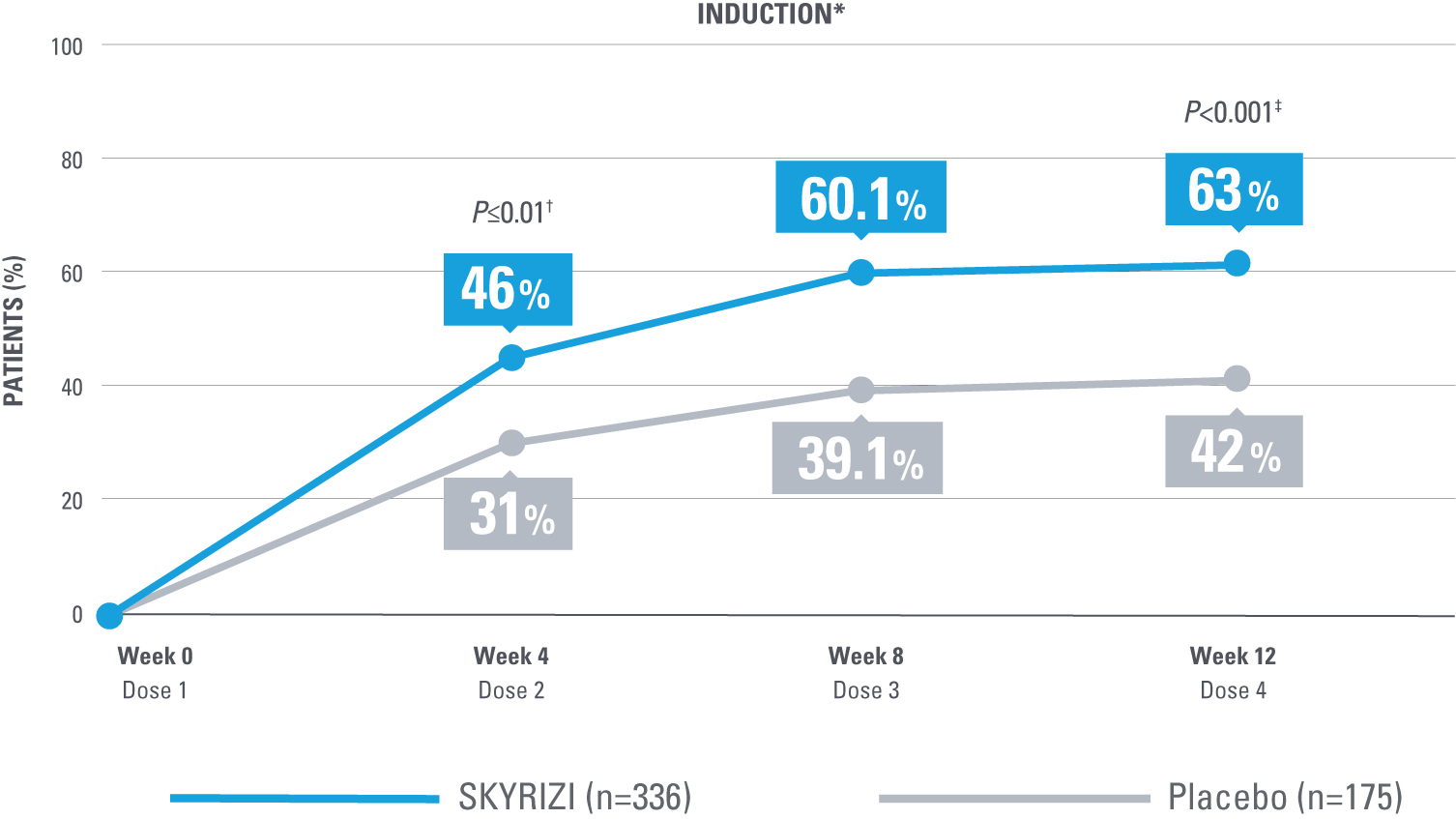
SIGNIFICANT CLINICAL RESPONSE AND CLINICAL REMISSION
After the first dose of SKYRIZI vs placebo (week 4)1*
ADVANCE: Enhanced Clinical Response at Weeks 4, 8, and 12 of Induction1,2,6
*ADVANCE Mixed Populations Study.
†Statistically significant under multiplicity control for SKYRIZI vs placebo (P≤0.01).1
‡Statistically significant under multiplicity control for SKYRIZI vs placebo (P<0.001).1
Enhanced clinical response at Weeks 4 and 12 was considered ranked secondary endpoints in the ADVANCE study. Enhanced clinical response at Week 8 was nominal vs placebo comparison. No clinical inferences can be drawn. Enhanced clinical response at Week 8 was derived from a post hoc analysis, and because this analysis was not powered or tested to demonstrate a significant difference in treatment effect, no statistical inferences can be made at Week 8.1,2
Enhanced clinical response (SF/APS): ≥60% decrease in average daily SF and/or ≥35% decrease in average daily APS and both not worse than baseline, and/or clinical remission.2
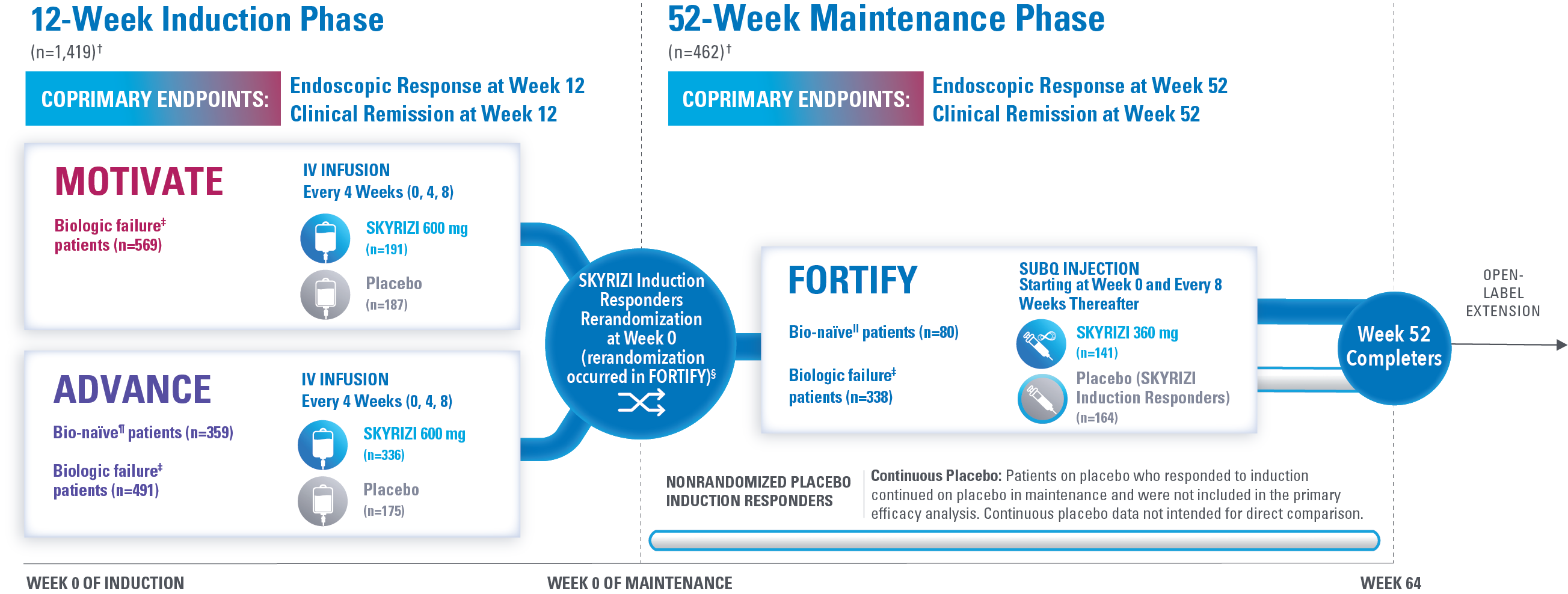
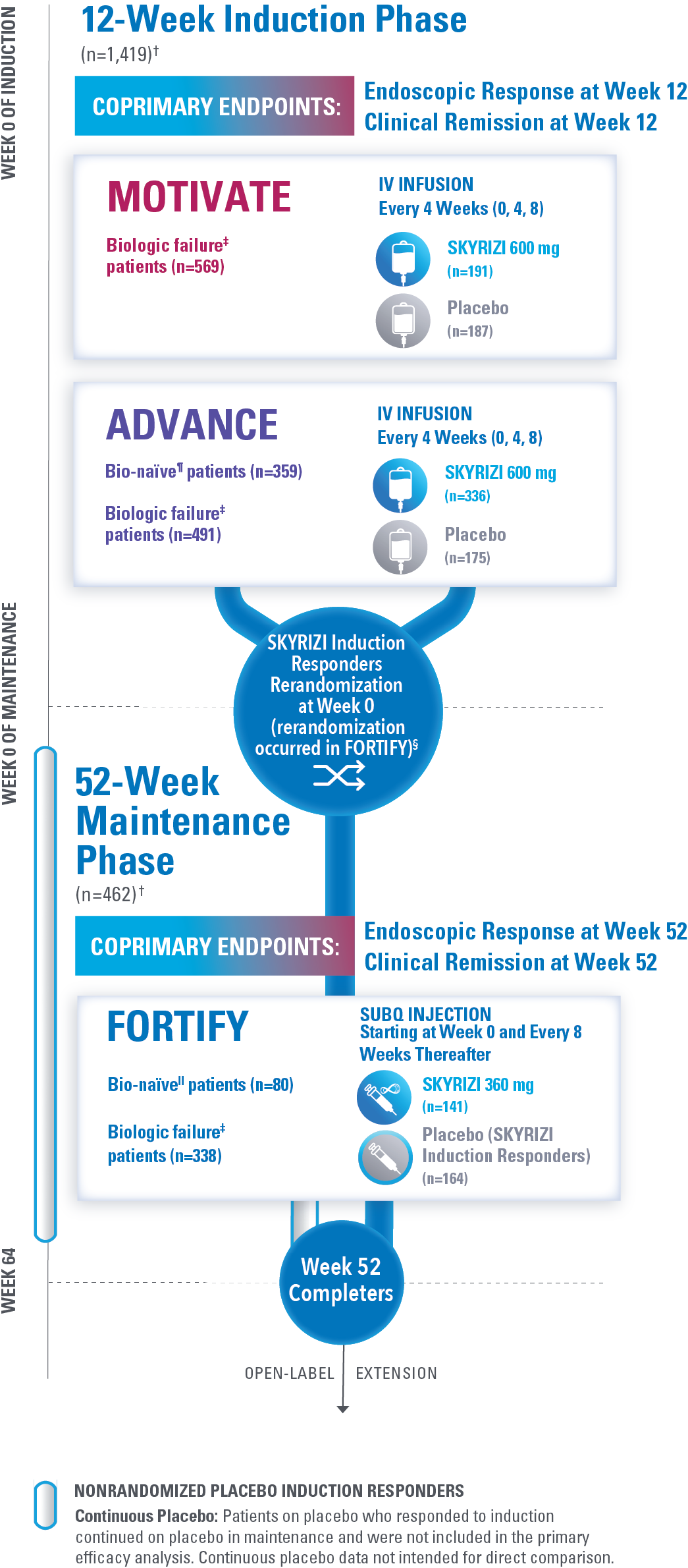
STUDY DESIGN AND BASELINE CHARACTERISTICS
Largest Completed Phase 3 Clinical Program in Crohn’s Disease to Date*
(Over 1,400 Patients in Treatment Arms)1-3
*As of February 2022.
†The total population in the MOTIVATE and ADVANCE studies includes patients dosed with risankizumab 1200 mg IV induction, which is not an approved dose for Crohn’s disease. The total population in the FORTIFY study includes patients dosed with risankizumab 1200 mg IV induction and risankizumab 180 mg SubQ maintenance, which are not approved doses for Crohn’s disease.1
‡The biologic failure subpopulation included patients who had failed or were intolerant to treatment with one or more biologic therapies.1
§Clinical response: ≥30% decrease in average daily SF and/or ≥30% decrease in average daily APS and both not worse than baseline.1
IIAmong the patients without prior biologic failure, at FORTIFY Week 0, 93% were naive to biologic therapy and the remaining 7% had received a biologic but never failed or demonstrated intolerance.4
¶Among the subjects without prior biologic failure, 87% were naïve to biologic therapy and the remaining 13% had received a biologic but never failed or demonstrated intolerance.1
Coprimary Endpoint Definitions
Endoscopic response: Decrease in SES-CD >50% from baseline (or a decrease of at least 2 points for subjects with a baseline score of 4 and isolated ileal disease).1
Clinical remission (SF/APS): Average daily stool frequency (SF) 2.8 and not worse than baseline and average daily abdominal pain score (APS) 1 and not worse than baseline.1
FULL INFORMATION ON ANALYZED PATIENT POPULATION1-3
The total population in the MOTIVATE and ADVANCE studies includes patients dosed with risankizumab 1200 mg IV induction, which is not an approved dose for Crohn’s disease. The total population in the FORTIFY study includes patients dosed with risankizumab 1200 mg IV induction and risankizumab 180 mg SubQ maintenance, which are not approved doses for Crohn’s disease.
In ADVANCE and MOTIVATE, efficacy was analyzed in the intent-to-treat (ITT) population, which included 1,419 randomized patients who received at least 1 study drug dose for the first 12-week induction period and had a baseline eligible SES-CD of ≥6 (≥4 for isolated ileal disease). Safety was analyzed in all randomized patients who received at least 1 study drug dose in the first 12-week period, including patients with a low SES-CD. Patients from 1 noncompliant site were excluded from efficacy but included in the safety analyzes (5 patients in ADVANCE, 13 in MOTIVATE).
In FORTIFY, the primary efficacy population included rerandomized clinical responders to 12 weeks of risankizumab IV induction who received at least 1 dose of study drug during the 52-week maintenance period and enrolled in the induction studies and who had an SES-CD of ≥6 (≥4 for isolated ileal disease) excluding the narrowing component. Additionally, the primary safety population included randomized patients who received 24 weeks of risankizumab during the induction studies including induction period 2, patients from a noncompliant site, and patients with a low SES-CD at induction baseline, as well as nonrandomized patients.
APS=abdominal pain score; IV=intravenous; SES-CD=Simple Endoscopic Score for Crohn’s Disease; SF=stool frequency; SubQ=subcutaneous.
Indication1
SKYRIZI is indicated for the treatment of adult patients with moderately to severely active Crohnʼs disease who have had an inadequate response to, lost response to, or were intolerant to conventional therapy or a biologic therapy.
Important Safety Information1
Affiliate to insert local ISI.
References: 1. SKYRIZI [Summary of Product Characteristics]. Ludwigshafen, Germany: AbbVie Deutschland GmbH & Co; January 2024. 2. D’Haens G, Panaccione R, Baert F, et al. Risankizumab as induction therapy for Crohn’s disease: results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet. 2022;399(10340):2015-2030. doi:10.1016/S0140-6736(22)00467-6 3. Ferrante M, Panaccione R, Baert F, et al. Risankizumab as maintenance therapy for moderately to severely active Crohn’s disease: results from the multicentre, randomised, double-blind, placebo-controlled, withdrawal phase 3 FORTIFY maintenance trial. Lancet. 2022;399(10340):2031-2046. doi:10.1016/S0140-6736(22)00466-4 4. Data on file, AbbVie Inc. ABVRRTI73681. 5. Data on file, AbbVie Inc. ABVRRTI73677. 6. Schreiber SW, Ferrante M, Panaccione R, et al. Risankizumab induces early clinical remission and response in patients with moderate-to-severe Crohn’s disease: results from the phase 3 ADVANCE and MOTIVATE studies. J Crohns Colitis. 2021;15(suppl 1):S026-S027. European Crohn’s and Colitis Organisation abstract OP26. doi:10.1093/ecco-jcc/jjab075.025