Note to affiliate: Consider the placement of the localized SEQUENCE video on this page.
SEQUENCE TRIAL
PRIMARY ENDPOINTS IN SEQUENCE
Clinical Remission at Week 24
(Non-inferiority of SKYRIZI vs ustekinumab)2*
Endoscopic Remission at Week 48
(Superiority of SKYRIZI vs ustekinumab)2
Note to affiliate: This statement is not inclusive of all secondary endpoints.
*The primary endpoint of clinical remission at Week 24 was set up to test non-inferiority and was evaluated in 50% of the patients.2
Endpoint Definitions
CDAI clinical remission: CDAI <150.2
Endoscopic remission: SES-CD ≤4 and at least a 2-point reduction vs baseline and no subscore greater than 1 in any individual variable, as scored by a central reviewer.2
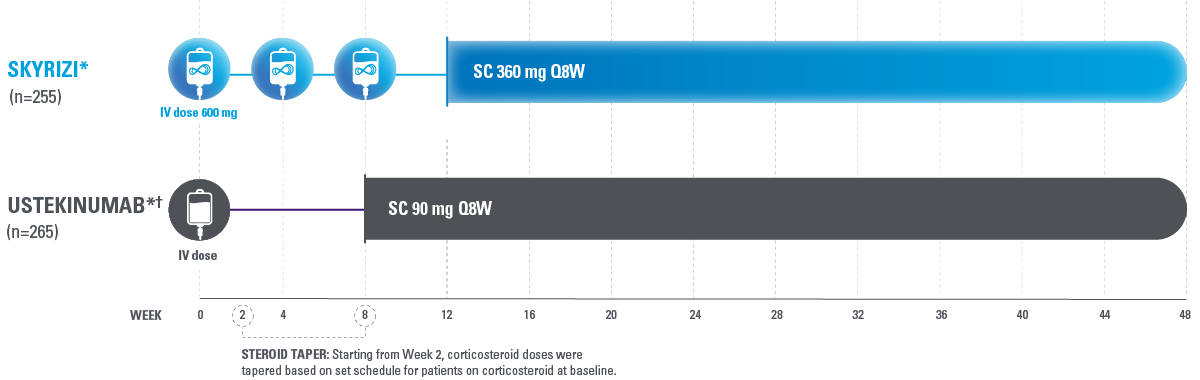
HEAD-TO-HEAD STUDY DESIGN
SEQUENCE was a Phase 3b, open-label, multicenter, randomized, efficacy assessor-blinded study of SKYRIZI compared with ustekinumab for the treatment of adult patients with moderately to severely active Crohn’s disease who have failed anti-TNF therapy.2
Site and patient were blinded to CDAI, and central endoscopy reader was blinded to study treatment.2
*Intent-to-treat 1 (ITT-1) population included all randomized subjects who were randomized to SKYRIZI with the selected SKYRIZI dose (SKYRIZI 600 mg IV followed by SKYRIZI 360 mg SC) or ustekinumab and who received at least 1 dose of study drug during Part 1 of the study.2
†Ustekinumab baseline IV dose is weight-based: ≤55 kg 260 mg dose, >55 kg to 85 kg 390 mg dose or >85 kg 520 mg.3
CDAI=Crohn’s Disease Activity Index; IV=intravenous; Q8W=every 8 weeks; SC=subcutaneous; TNF=tumor necrosis factor.
HEAD-TO-HEAD STUDY DETAILS
SEQUENCE was a Phase 3b, open-label, multicenter, randomized, efficacy assessor-blinded study of SKYRIZI compared with ustekinumab for the treatment of adult patients with moderately to severely active Crohn’s disease who have failed anti-TNF therapy.2
Primary Endpoints (sequential testing)2
- CDAI clinical remission, CDAI <150, Week 24 (50% population), non-inferiority (10% margin), iDBL1
- Endoscopic remission, Week 48 (100% population), superiority, iDBL3, level of 0.05 (2-sided)
Ranked Secondary Endpoints (superiority, 100% population, iDBL3)2
- CDAI clinical remission, CDAI <150 Week 48
- Endoscopic response Week 48
- Endoscopic response Week 24
- Steroid-free endoscopic remission Week 48
- Steroid-free clinical remission at Week 48
Key Eligibility2:
- CDAI 220-450
- SF ≥4.0 and/or APS ≥2.0
- SES-CD ≥6 or ≥4 for isolated ileal disease (excluding the narrowing component) as scored by blinded central reviewer
- Demonstrated intolerance or inadequate response to 1 or more anti-TNF therapies
Stratification Factors2:
- Number of prior anti-TNF failure (1, >1)
- Corticosteroid use at baseline (yes or no)
Steroid-free endoscopic remission: endoscopic remission and not receiving steroids at the corresponding visit.2
Steroid-free clinical remission: clinical remission and not receiving steroids at the corresponding visit.2
Intent-to-treat-1 (ITT-1) population included all randomized subjects who were randomized to SKYRIZI with the selected SKYRIZI dose (SKYRIZI 600 mg IV followed by SKYRIZI 360 mg SC) or ustekinumab and who received at least 1 dose of study drug during Part 1 of the study.
After ADVANCE and MOTIVATE Phase 3 study results were available, the SKYRIZI dosing regimen was adjusted to 600 mg IV for induction and 360 mg SC for maintenance (“selected dosing regimen”).2
Overall Type I error rate was strongly controlled at 2-sided alpha level of 0.05 by the fixed-sequence multiplicity control method for this study.
APS=abdominal pain score; CDAI=Crohn’s Disease Activity Index; iDBL=interim database lock; IR=intolerance or inadequate response; IV=intravenous; SC=subcutaneous; SES-CD=simple endoscopic score for Crohnʼs disease; SF=stool frequency; TNF=tumor necrosis factor.
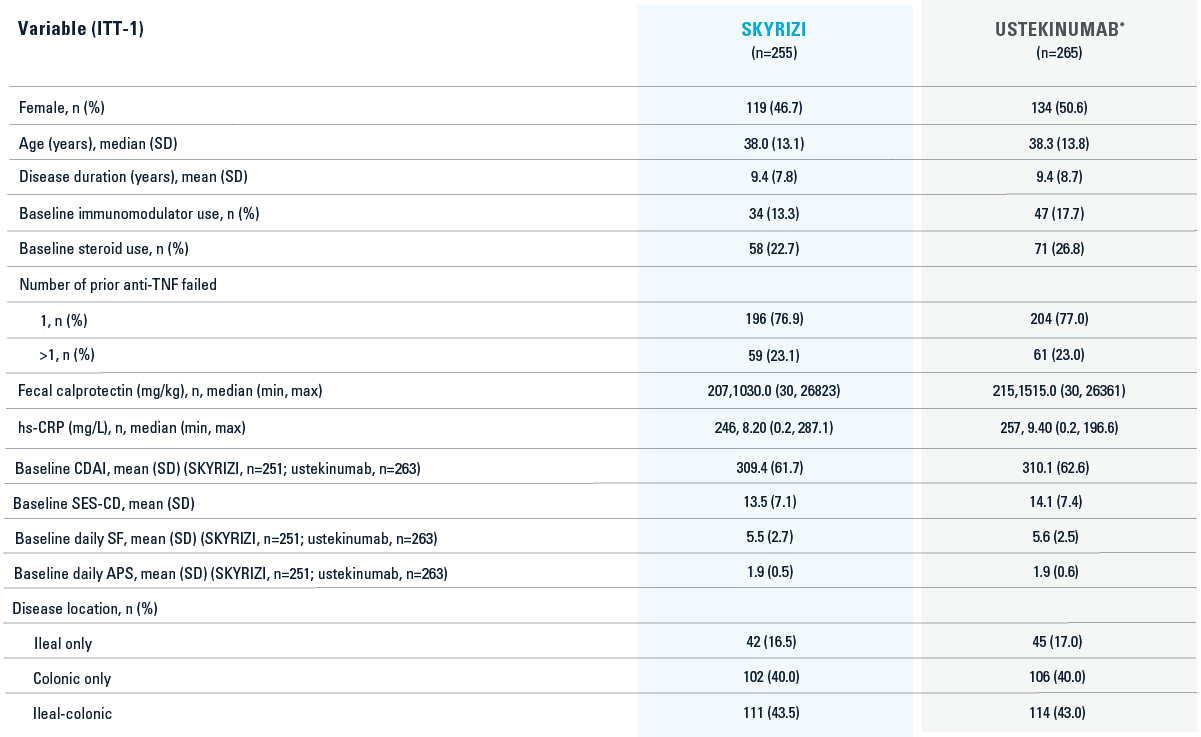
HEAD-TO-HEAD STUDY BASELINE CHARACTERISTICS3
*Ustekinumab baseline IV dose is weight-based: ≤55 kg 260 mg dose, >55 kg to 85 kg 390 mg dose or >85 kg 520 mg.3
Intent-to-treat-1 (ITT-1) population included all randomized subjects who were randomized to SKYRIZI with the selected SKYRIZI dose (SKYRIZI 600 mg IV followed by SKYRIZI 360 mg SC) or ustekinumab and who received at least 1 dose of study drug during Part 1 of the study.
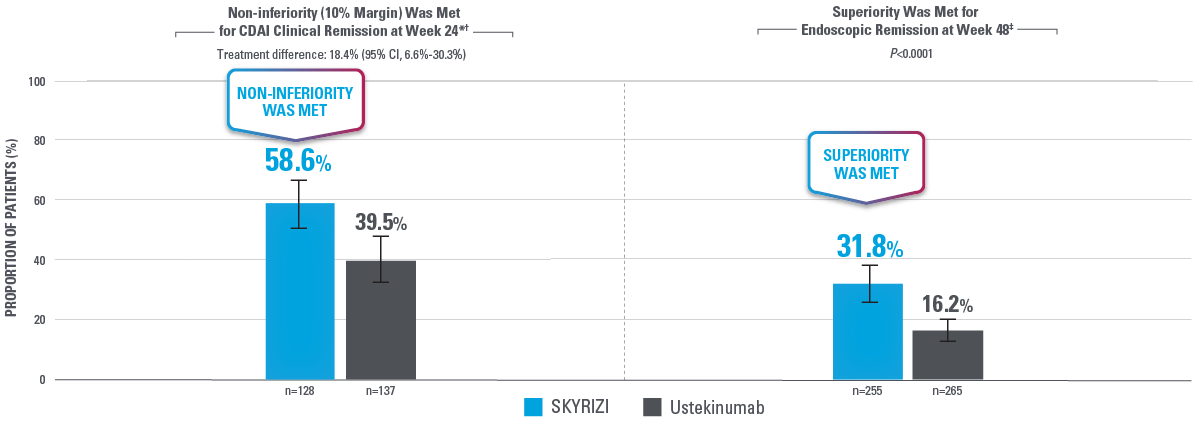
BOTH PRIMARY ENDPOINTS WERE MET2
*Clinical remission at Week 24 was measured by non-inferiority in 50% of subjects. Non-inferiority endpoints aim to demonstrate whether the effect of 1 intervention is no worse than an active intervention.2
†ITT1H: Analysis population that was a subset of the ITT-1 population and included approximately 50% of ITT-1 patients who had an opportunity to reach Week 24 by the time of primary analysis of CDAI clinical remission (non-inferiority of risankizumab vs ustekinumab) at Week 24.2
‡Intent-to-treat 1 (ITT-1) population included all randomized subjects who were randomized to SKYRIZI with the selected SKYRIZI dose (SKYRIZI 600 mg IV followed by SKYRIZI 360 mg SC) or ustekinumab and who received at least 1 dose of study drug during Part 1 of the study.2
Error bars: 95% CI.2
CDAI clinical remission: CDAI <150.2
Endoscopic remission: SES-CD ≤4 and at least a 2-point reduction vs baseline and no subscore greater than 1 in any individual variable, as scored by a central reviewer.2
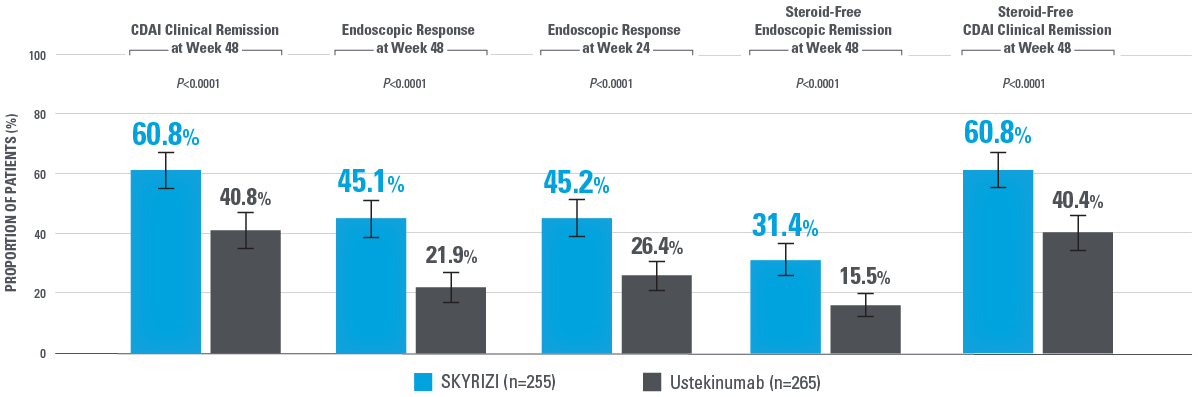
SKYRIZI ACHIEVED SUPERIORITY IN ALL RANKED SECONDARY ENDPOINTS COMPARED WITH USTEKINUMAB2
Note to affiliate: Steroid-free endoscopic remission is not in the SmPC. Evaluate uses of these data per your local regulatory code.
*Ranked secondary endpoints are presented by order of testing.2
Error bars: 95% CI.2
Overall Type I error rate was strongly controlled at 2-sided alpha level of 0.05 by the fixed-sequence multiplicity control method for this study.
Intent-to-treat 1 (ITT-1) population included all randomized subjects who were randomized to SKYRIZI with the selected SKYRIZI dose (SKYRIZI 600 mg IV followed by SKYRIZI 360 mg SC) or ustekinumab and who received at least 1 dose of study drug during Part 1 of the study.2
CDAI clinical remission: CDAI <150.2
Endoscopic remission: SES-CD ≤4 and at least a 2-point reduction vs baseline and no subscore >1 in any individual variable.2
Endoscopic response: Decrease in SES-CD >50% from baseline (or for patients with isolated ileal disease and a baseline SES-CD of 4, at least a 2-point reduction from baseline), as scored by central reviewer.2
Steroid-free endoscopic remission: Endoscopic remission and not receiving steroids at the corresponding visit.2
Steroid-free clinical remission: Clinical remission and not receiving steroids at the corresponding visit.2
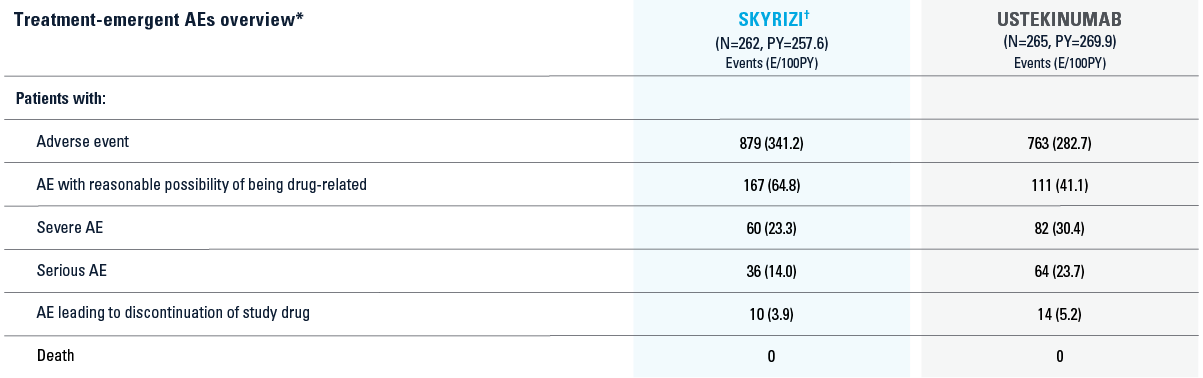
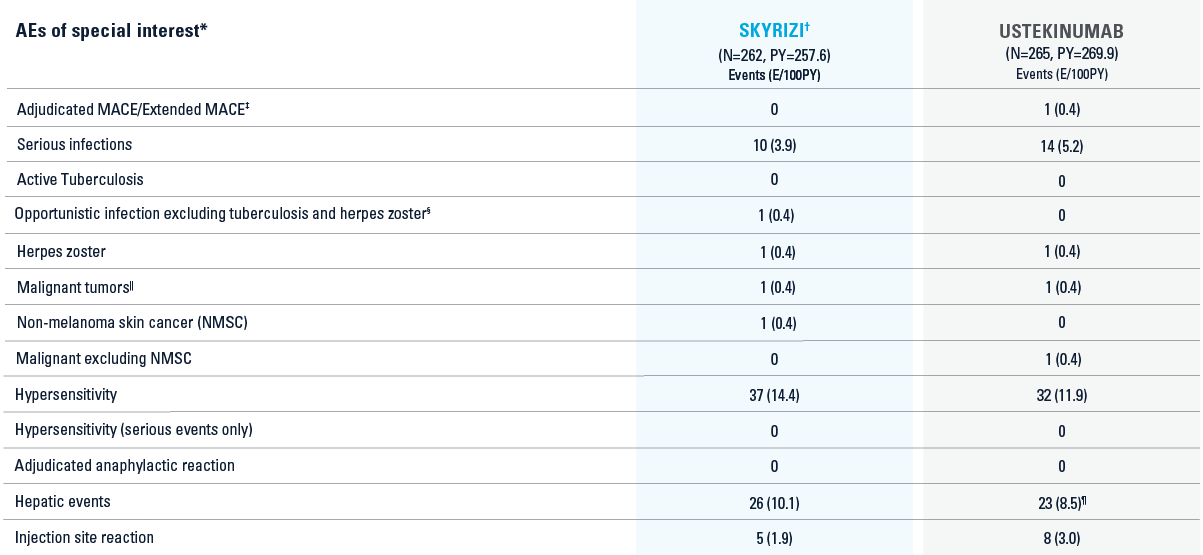
SAFETY DATA FROM SEQUENCE TRIAL2
*SA1: Includes all patients who were randomized and received at least one dose of study drug.3
†Includes patients who received 1200 mg induction before the label dose was approved. Those who received 1200 mg are not included for efficacy, but are included in the safety analyses.
*SA1: Includes all patients who were randomized and received at least one dose of study drug.3
†Includes patients who received 1200 mg induction before the label dose was approved. Those who received 1200 mg are not included for efficacy, but are included in the safety analyses.
‡MACE: Defined as cardiovascular death or death due to stroke, nonfatal myocardial infarction, and nonfatal stroke. Extended MACE: Defined as MACE with hospitalization for unstable angina and coronary revascularization procedures.
§Opportunistic infection: SKYRIZI, esophageal candidiasis.
||Malignant tumor: SKYRIZI, squamous cell carcinoma of skin; ustekinumab, anal squamous cell carcinoma.
¶One case of potential Hy’s law in the ustekinumab treatment group.
AE=adverse event; MACE=major adverse cardiovascular events; PY=patient-year.
Indication1
SKYRIZI is indicated for the treatment of adult patients with moderately to severely active Crohnʼs disease who have had an inadequate response to, lost response to, or were intolerant to conventional therapy or a biologic therapy.
Important Safety Information1
Affiliate to insert local ISI.
References: 1. SKYRIZI [Summary of Product Characteristics]. Ludwigshafen, Germany: AbbVie Deutschland GmbH & Co; January 2024. 2. Peyrin-Biroulet L, Chapman JC, Colombel JF, et al. Risankizumab versus ustekinumab for patients with moderate to severe Crohn’s disease: results from the phase 3b SEQUENCE study. Abstract presented at: United European Gastroenterology Week (UEGW); October 14-17, 2023; Copenhagen, Denmark. 3. Peyrin-Biroulet L, Chapman JC, Colombel JF, et al. Risankizumab versus ustekinumab for patients with moderate to severe Crohn’s disease: results from the phase 3b SEQUENCE study. Oral presentation at: United European Gastroenterology Week (UEGW); October 14-17, 2023; Copenhagen, Denmark. 4. Ferrante M, Panaccione R, Baert F, et al. Risankizumab as maintenance therapy for moderately to severely active Crohn’s disease: results from the multicentre, randomised, double-blind, placebo-controlled, withdrawal phase 3 FORTIFY maintenance trial. Lancet. 2022;399(10340):2031-2046. doi:10.1016/S0140-6736(22)00466-4