RINVOQ® (upadacitinib) is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis (UC) who have had an inadequate score, lost response or were intolerant to either conventional therapy or a biologic agent.1
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
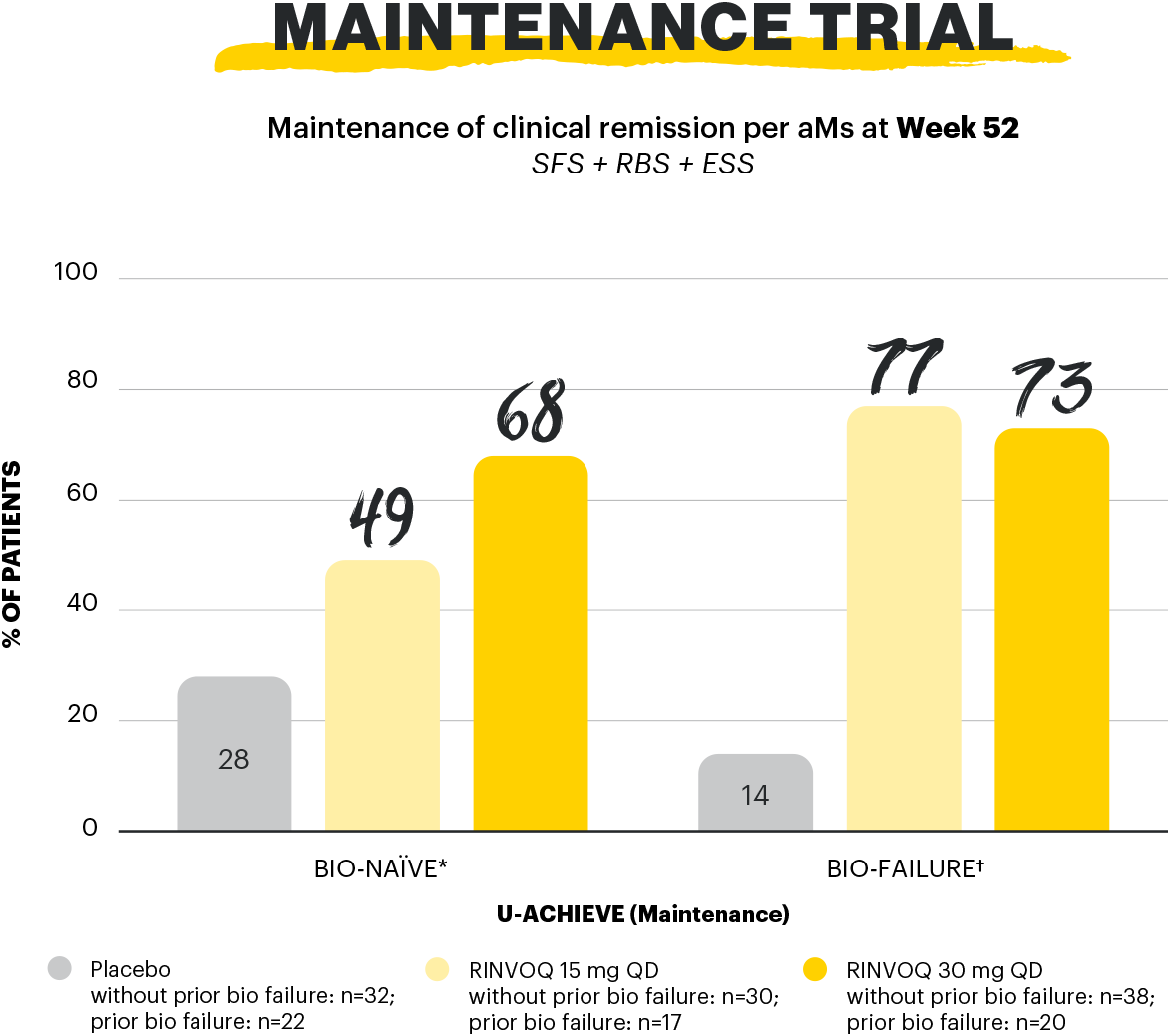
*Bio-naïve defined as without prior biologic failure and includes patients who had previous exposure to biologics but did not experience treatment failure.
†Bio-failure refers to prior treatment failure to at least 1 biologic therapy.
Maintenance of clinical remission: clinical remission per aMs at Week 52 among patients who achieved clinical remission per aMs at Week 8 of RINVOQ 45 mg QD induction treatment (n=159).2
aMs: adapted Mayo score; ESS: endoscopic subscore; QD: once daily; RBS: rectal bleeding subscore; SFS: stool frequency subscore.
REFERENCES
- RINVOQ Summary of Product Characteristics.
- Danese S, Vermeire S, Zhou W, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399(10341):2113–2128.