RINVOQ® (upadacitinib) is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis (UC) who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.1
RINVOQ achieved the primary endpoints of clinical remission per adapted Mayo score at Induction Week 8 and Maintenance Week 521,2
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
A Phase 3 trial program involving 3 studies: 2 replicate induction studies (U-ACHIEVE Induction and U-ACCOMPLISH) and 1 maintenance study (U-ACHIEVE Maintenance). A total of 988 patients with moderately to severely active UC evaluating RINVOQ 45 mg QD vs placebo for induction and RINVOQ 15 mg QD and 30 mg QD vs placebo for maintenance treatment (N=451).1*
*Patients who achieved clinical response per adapted Mayo score with 8-week RINVOQ 45 mg QD induction treatment entered maintenance.
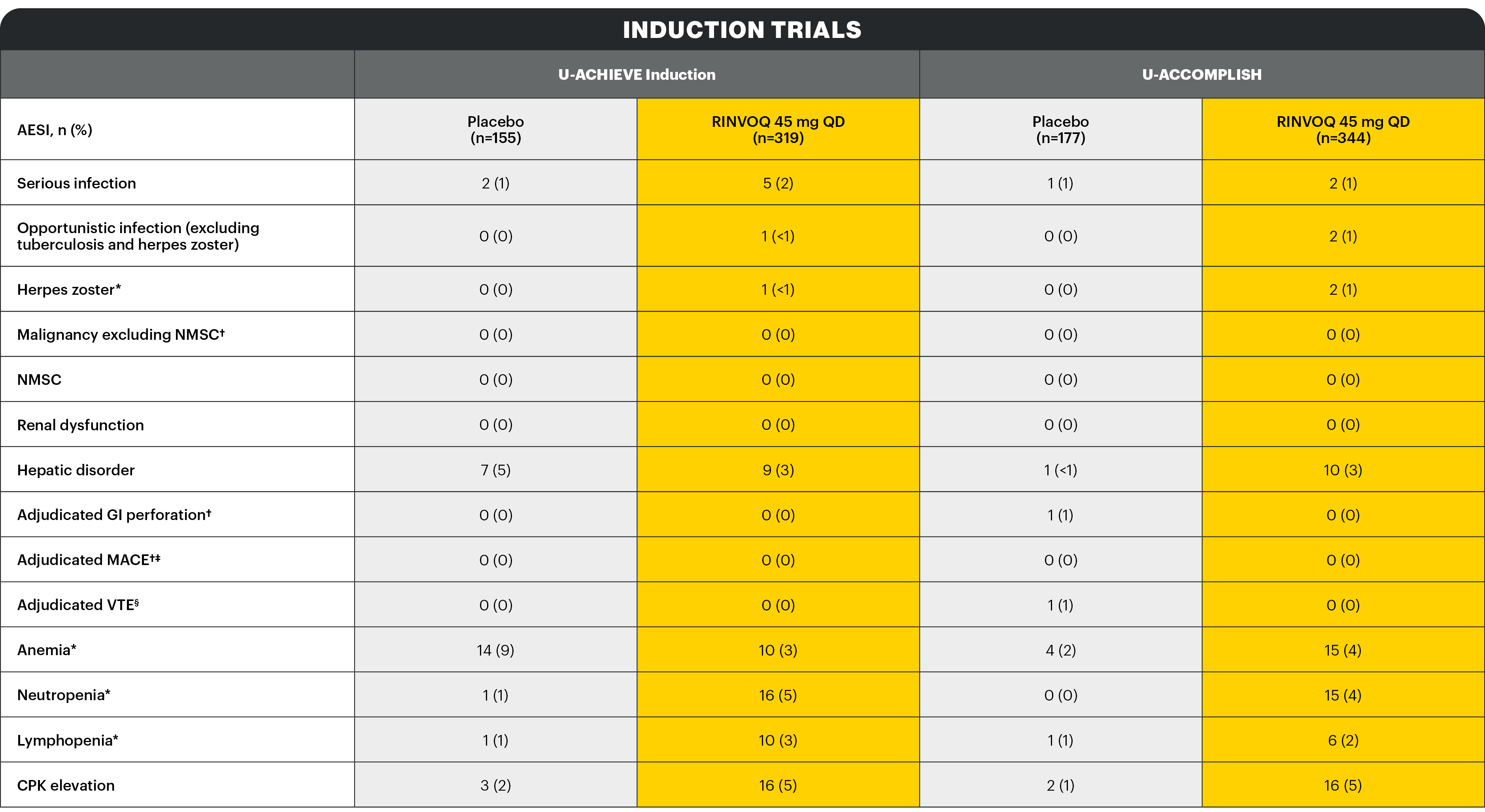
*Search criteria were based on Company MedDRA Query. †These events were determined on the basis of external adjudication. ‡MACE is defined as cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke. §VTE is defined as deep vein thrombosis and pulmonary embolism (fatal and non-fatal).
There were no AESIs of active tuberculosis or lymphoma in the study.
Serious infection1
The most frequently reported serious infection in UC studies was COVID-19 pneumonia.
Herpes zoster2
The majority of herpes zoster events observed with RINVOQ were mono-dermatomal and uncomplicated.
One event led to RINVOQ discontinuation.
CPK elevation2
No events of CPK were serious and most were asymptomatic. Most events were mild or moderate in severity and assessed by investigator as possibly related to study drug. Discontinuation of RINVOQ due to CPK elevation was infrequent.
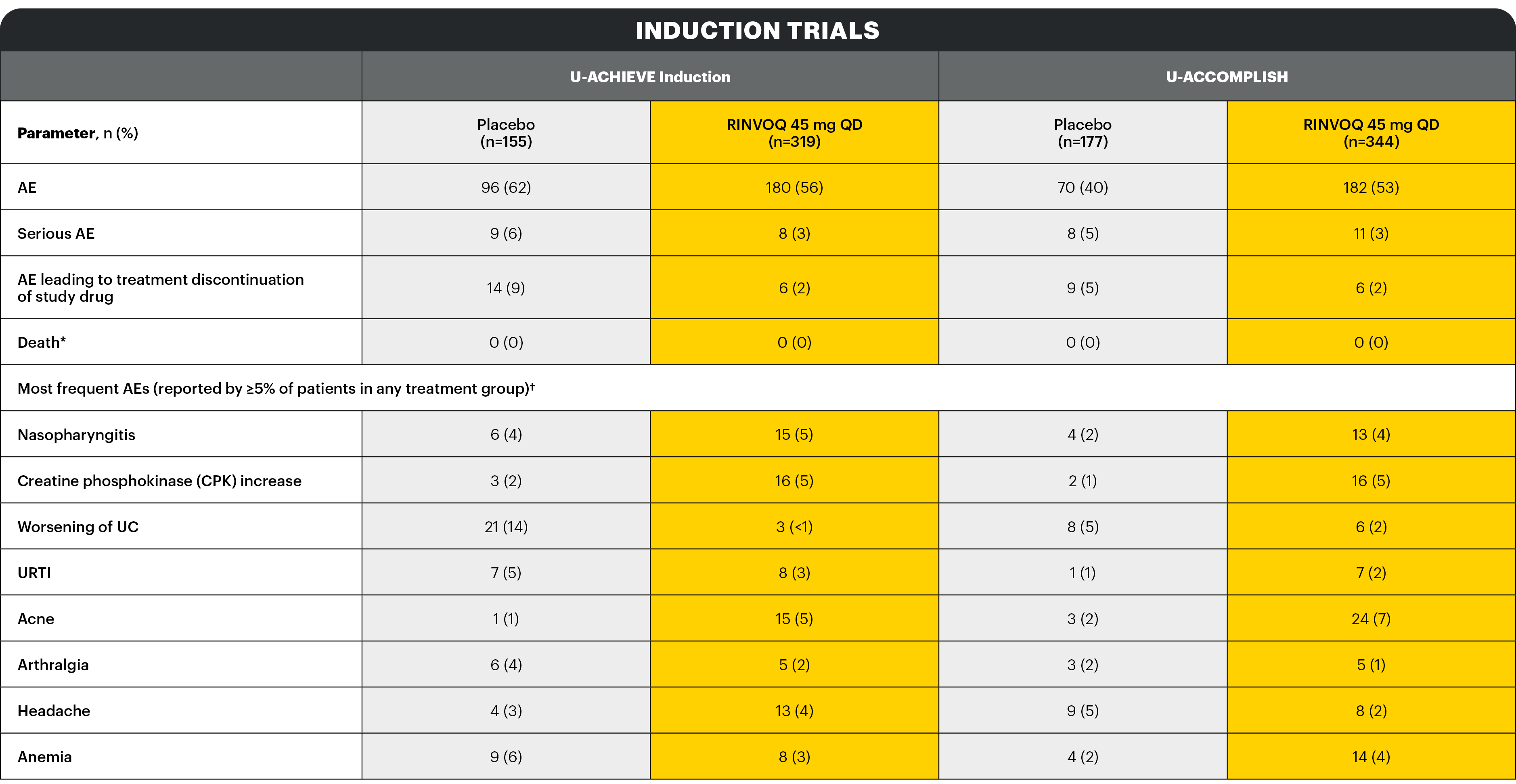
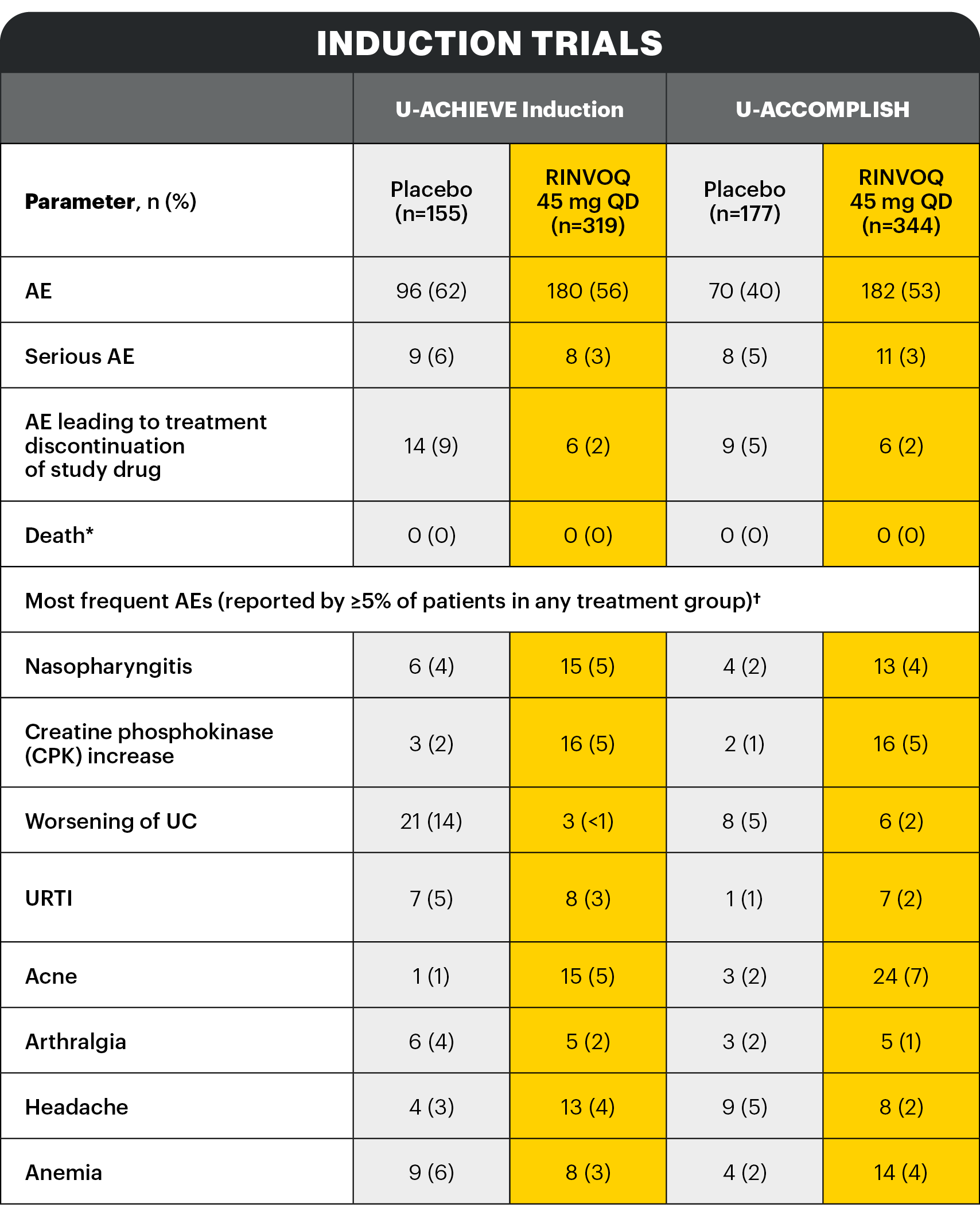
AESI: Infections1
Description of selected adverse reactions in the placebo-controlled induction studies:
Infections1
The frequency of infection over 8 weeks in the RINVOQ 45 mg QD group compared to the placebo group was 20.7% and 17.5%, respectively.
The frequency of serious infection over 8 weeks in both the RINVOQ 45 mg QD group and the placebo group was 1.3%. No additional serious infections were observed over 8-week extended treatment with RINVOQ 45 mg QD.
Opportunistic infections (excluding tuberculosis)1
In the placebo-controlled induction studies over 8 weeks, the frequency of opportunistic infection (excluding tuberculosis and herpes zoster) in the RINVOQ 45 mg QD group was 0.4% and 0.3% in the placebo group. No additional opportunistic infections (excluding tuberculosis and herpes zoster) were observed over 8-week extended treatment with RINVOQ 45 mg QD.
In the placebo-controlled induction studies over 8 weeks, the frequency of herpes zoster in the RINVOQ 45 mg QD group was 0.6% and 0% in the placebo group. The frequency of herpes zoster was 3.9% over 16-week treatment with RINVOQ 45 mg QD.
Extended induction treatment1
No additional serious or opportunistic infections (excluding tuberculosis and herpes zoster) were observed over 8‑week extended treatment with RINVOQ 45 mg QD.
A higher rate of herpes zoster was observed with an induction treatment period of 16 weeks vs 8 weeks: the frequency of herpes zoster was 3.9% over 16-week treatment with RINVOQ 45 mg QD.
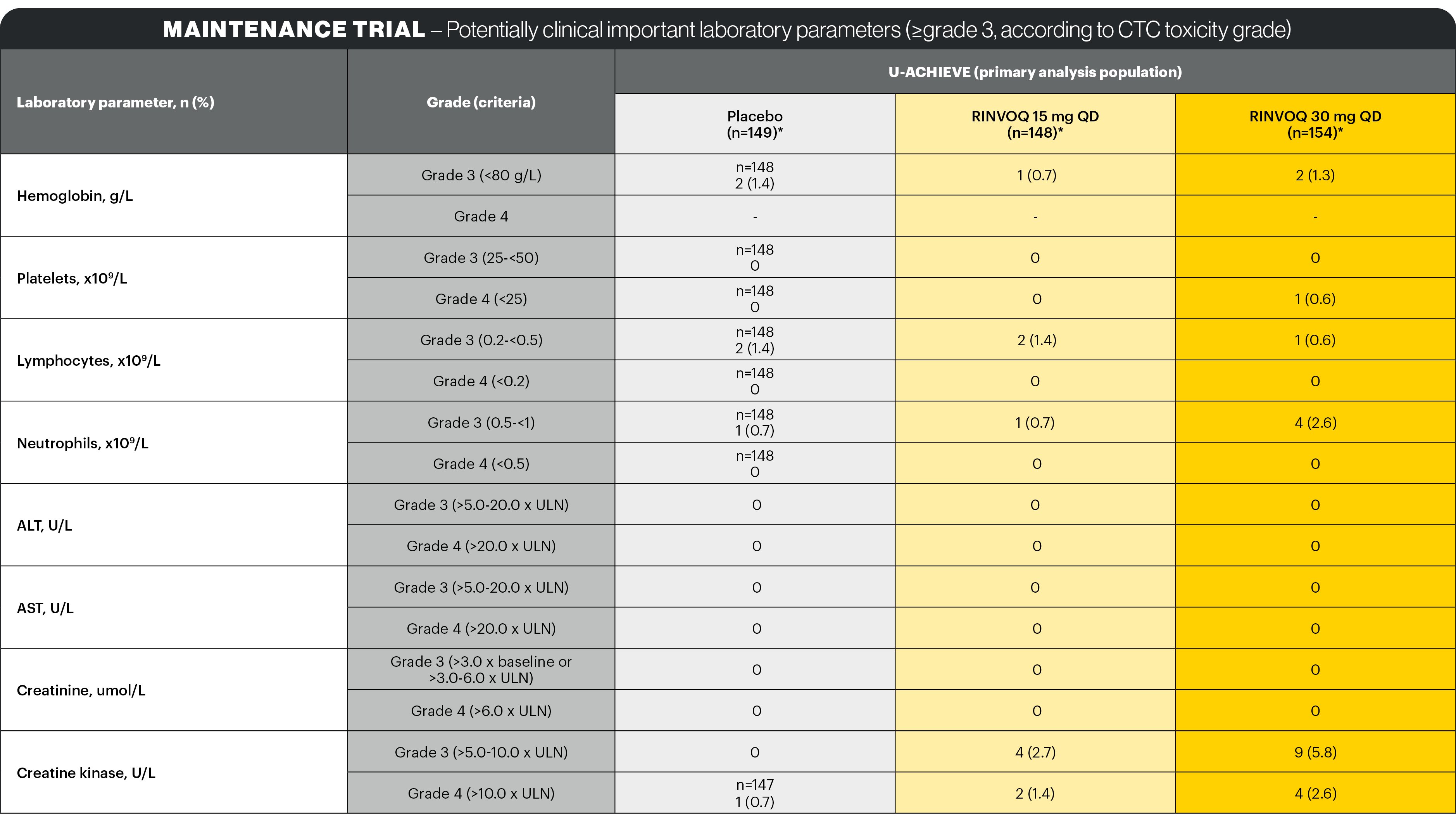
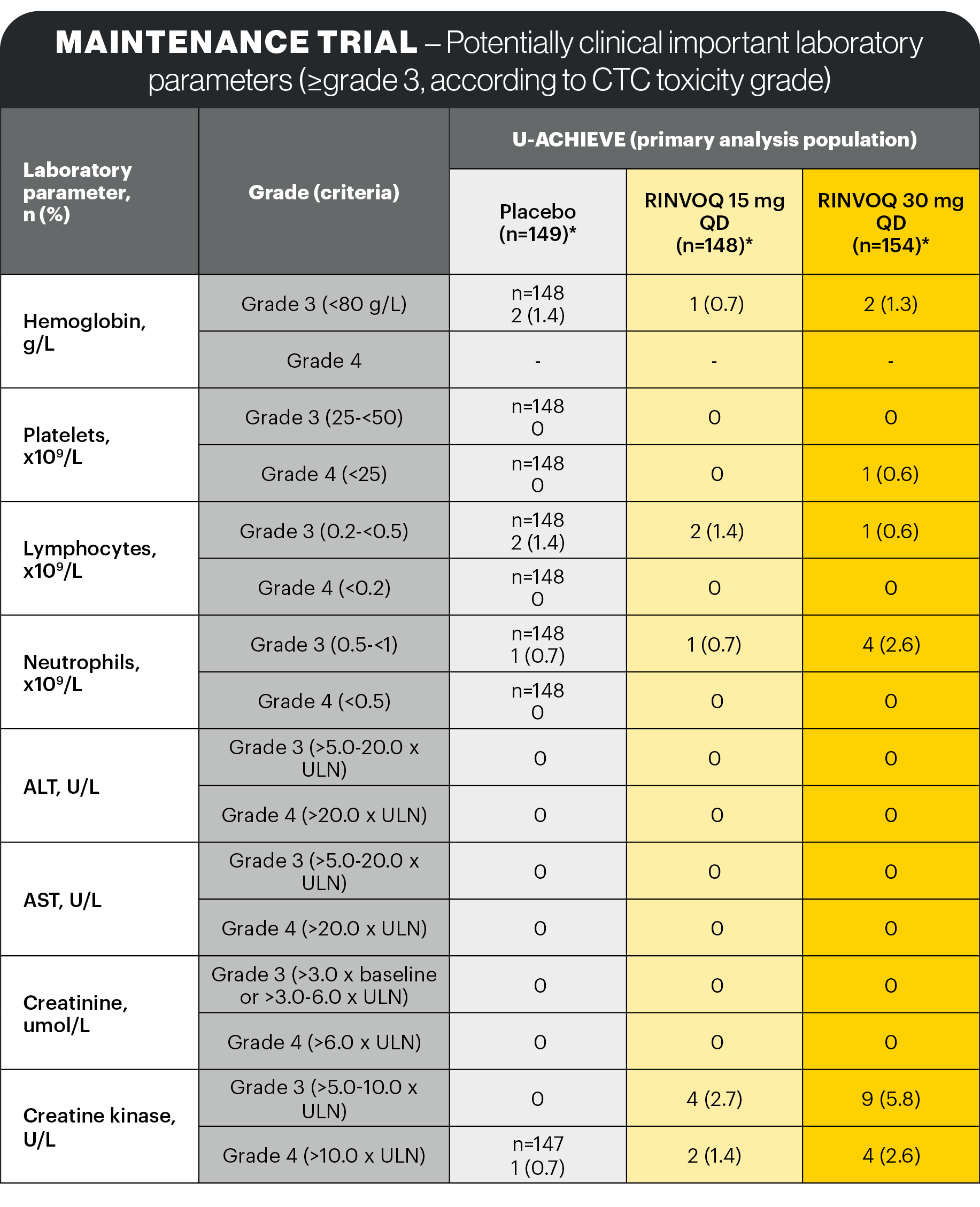
Lab abnormalities1
In the placebo-controlled 8-week induction and 52-week maintenance clinical studies, the laboratory changes in ALT increased and/or AST increased (≥3 x ULN), CPK values (>5 x ULN), and neutropenia (ANC <1 x 109 cells/L) associated with RINVOQ treatment were generally similar to what was observed in the rheumatologic disease and AD clinical studies.
Dose dependent changes for these laboratory parameters associated with 15 mg QD and 30 mg QD RINVOQ treatment were observed.
In the placebo-controlled induction studies for up to 8 weeks, decreases in lymphocyte counts below 0.5 x 109 cells/L in at least one measurement occurred in 2.0% and 0.8% of patients in the RINVOQ 45 mg QD and placebo groups, respectively.
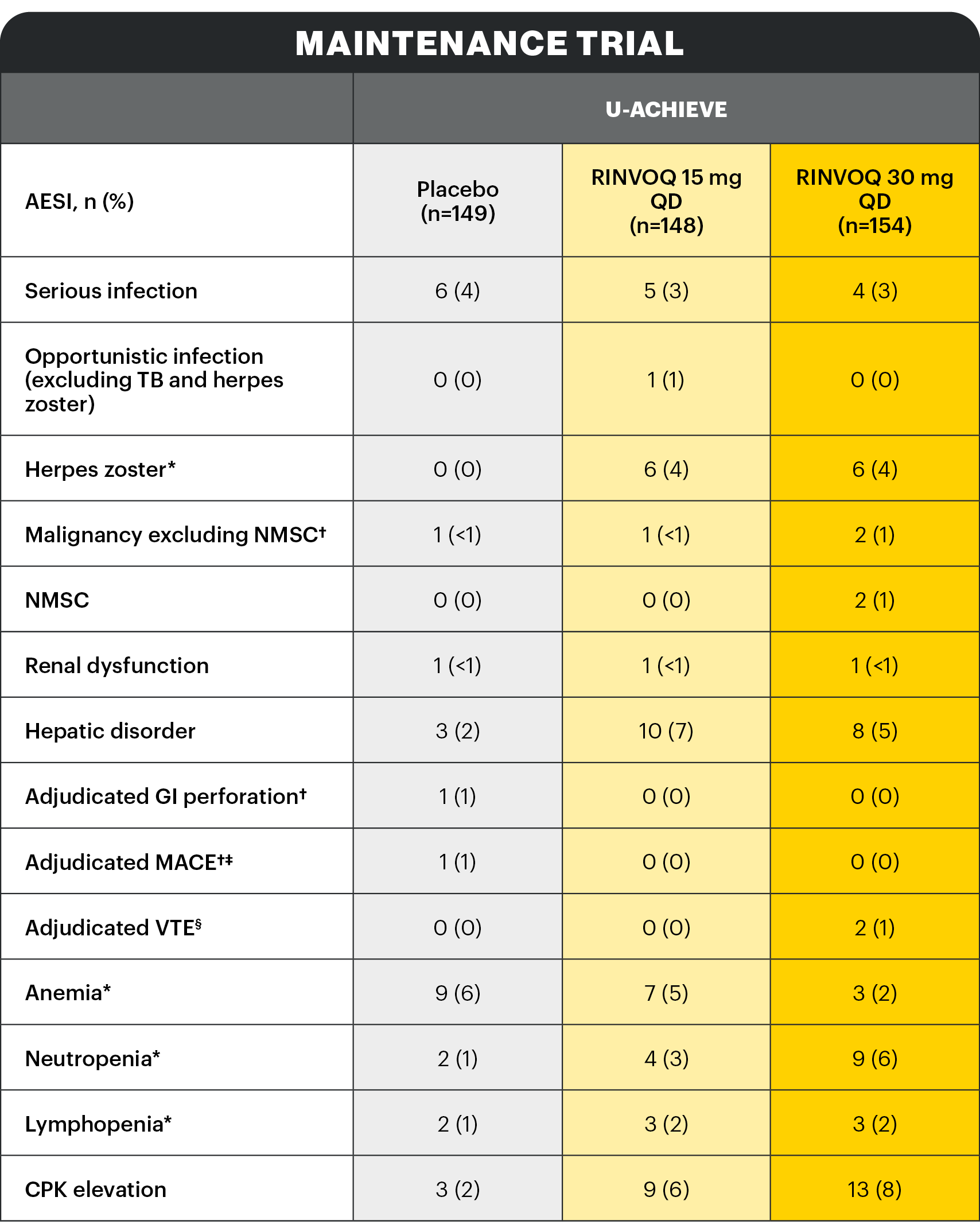
*Search criteria were based on Company MedDRA Query. †These events were determined on the basis of external adjudication. ‡MACE is defined as cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke. §VTE is defined as deep vein thrombosis and pulmonary embolism (fatal and non-fatal).
There were no AESIs of active tuberculosis or lymphoma in the study.
Serious infection1
The most frequently reported serious infection in UC studies was COVID-19 pneumonia.
Herpes zoster2
The majority of herpes zoster events observed with RINVOQ were mono-dermatomal and uncomplicated.
Few events led to RINVOQ discontinuation.
Malignancy excluding NMSC2
Two patients on 30 mg QD: colon cancer, prostate cancer. One patient on 15 mg QD: invasive breast cancer. One patient on placebo: invasive breast cancer.
Adjudicated VTE2
Patients with VTEs in RINVOQ Phase 3 UC studies had at least one VTE risk factor at baseline.
Two non-serious events of deep vein thrombosis (DVT) were reported in the RINVOQ 30 mg QD group; one event led to study drug discontinuation:
- 64-year-old male: right axillary vein thrombosis with concomitant serious COVID-19 pneumonia, acute respiratory failure, hypoxia, and diastolic congestive heart failure. DVT event not related to study drug.
- 74-year-old male: DVT of left popliteal vein, not related to study drug. Patient discontinued study drug due to event. Risk factor: high BMI.
CPK elevation2
No events of CPK were serious and most were asymptomatic. Most events were mild or moderate in severity and assessed by investigator as possibly related to study drug.
Discontinuation of RINVOQ due to CPK elevation was infrequent.
AESI: Infections1
Description of selected adverse reactions in the placebo-controlled maintenance study:
Infections1
The frequency of infection over 52 weeks in the RINVOQ 15 mg QD and 30 mg QD groups was 38.4% and 40.6%, respectively, compared to 37.6% in the placebo group. The long-term rate of infections for RINVOQ 15 mg QD and 30 mg QD was 73.8 and 82.6 events per 100 patient-years (PYs), respectively.
Serious infection1
The frequency of serious infection over 52 weeks in the RINVOQ 15 mg QD and 30 mg QD groups was 3.2% and 2.4%, respectively, compared to 3.3% in the placebo group. The long-term rate of serious infections for the RINVOQ 15 mg QD and 30 mg QD groups was 4.1 and 3.9 events per 100 PYs, respectively. The most frequently reported serious infection in the induction and maintenance phases was COVID-19 pneumonia.
Opportunistic infection1
The frequency of opportunistic infection (excluding tuberculosis and herpes zoster) in the RINVOQ 15 mg QD and 30 mg QD groups was 0.8% and 0.4%, respectively, compared to 0.8% in the placebo group. The long-term rate of opportunistic infections (excluding tuberculosis and herpes zoster) for the RINVOQ 15 mg QD and 30 mg QD groups was 0.6 and 0.3 events per 100 PYs, respectively.
Herpes zoster1
The frequency of herpes zoster in the RINVOQ 15 mg QD and 30 mg QD groups was 4.4% and 4.0%, respectively, compared to 0% in the placebo group. The long-term rate of herpes zoster for the RINVOQ 15 mg QD and 30 mg QD groups was 5.7 and 6.3 events per 100 PYs, respectively.
Lab abnormalities1
In the placebo-controlled maintenance study, for up to 52 weeks, decreases in lymphocyte counts below 0.5 x 109 cells/L in at least one measurement occurred in 1.6%, 0.8%, and 0.8% of patients in the RINVOQ 15 mg QD, 30 mg QD, and placebo groups, respectively. In clinical studies, treatment was interrupted in response to ALC <0.5 x 109 cells/L. No notable mean changes of lymphocyte counts were observed during RINVOQ treatment over time.
Elevations in lipid parameters were observed at 8 weeks of treatment with RINVOQ 45 mg QD and remained generally stable with longer-term treatment with RINVOQ 15 mg QD and 30 mg QD. Among patients in the placebo-controlled induction studies with baseline values below the specified limits, the following frequencies of patients were observed to shift to above the specified limits on at least one occasion during 8 weeks (including patients who had an isolated elevated value):
- Total cholesterol ≥5.17 mmol/L (200 mg/dL): 49% vs 11%, in the RINVOQ 45 mg QD and placebo groups, respectively
- LDL cholesterol ≥3.36 mmol/L (130 mg/dL): 27% vs 9%, in the RINVOQ 45 mg QD and placebo groups, respectively
- HDL cholesterol ≥1.03 mmol/L (40 mg/dL): 79% vs 36%, in the RINVOQ 45 mg QD and placebo groups, respectively
- Triglycerides ≥2.26 mmol/L (200 mg/dL): 6% vs 4%, in the RINVOQ 45 mg QD and placebo groups, respectively
The safety profile of RINVOQ was generally consistent with that observed in patients with rheumatoid arthritis1
AE: adverse event; AESI: adverse event of special interest; ALC: absolute lymphocytic count; ALT: alanine aminotransferase; aMs: adapted Mayo score; ANC: absolute neutrophil count; AST: aspartate aminotransferase; COVID-19: coronavirus disease 2019; CPK: creatine phosphokinase; CTC: common toxicity criteria; DVT: deep vein thrombosis; ESS: endoscopic subscore; GI: gastrointestinal; JAK: Janus kinase; MACE: major adverse cardiac event; NMSC: non-melanoma skin cancer; PY: patient-years; QD: once daily; RA: rheumatoid arthritis; RBS: rectal bleeding score; UC: ulcerative colitis; ULN: upper limit of the normal; URTI: upper respiratory tract infection; VTE: venous thromboembolism.
Study designs: U-ACHIEVE Induction (UC-1) and U-ACCOMPLISH (UC-2) were replicate induction studies, both of which were multicenter, double-blind, placebo-controlled clinical studies. In UC-1 and UC-2, 988 patients (473 and 515 patients, respectively) were randomized to RINVOQ 45 mg QD or placebo for 8 weeks with a 2:1 treatment allocation ratio and included in the efficacy analysis. All enrolled patients had moderately to severely active UC defined as aMs of 5 to 9 with an ESS of 2 or 3 and demonstrated prior treatment failure including inadequate response, loss of response, or intolerance to prior conventional and/or biologic treatment. U-ACHIEVE Maintenance (UC-3) was a multicenter, double-blind, placebo-controlled clinical study with 451 patients who achieved clinical response per aMs (decrease ≥2 points and ≥30% from baseline and a decrease in RBS ≥1 from baseline or an absolute RBS ≤1) with 8-week RINVOQ 45 mg QD induction treatment. Patients were rerandomized 1:1:1 to receive either RINVOQ 15 mg QD, 30 mg QD, or placebo.1,2
UP NEXT
RINVOQ is an oral, once daily, selective and reversible JAK inhibitor now approved for the treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.1
RINVOQ can be taken at any time of the day, with or without food. Tablets should be swallowed whole and should not be split, crushed, or chewed in order to ensure the entire dose is delivered correctly.1
A Phase 3 clinical trial program involving 3 studies: 2 replicate 8-week induction studies and 1 52-week maintenance study evaluated RINVOQ 45 mg QD vs placebo for induction and RINVOQ 15 mg QD and 30 mg QD vs placebo for maintenance treatment.1,2
[Please insert local summary of safety]
REFERENCES
- RINVOQ [Summary of Product Characteristics]. AbbVie Deutschland GmbH & Co. KG; December 2023.
- Danese S, Vermeire S, Zhou W, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399(10341):2113-2128. doi:10.1016/S0140-6736(22)00581-5