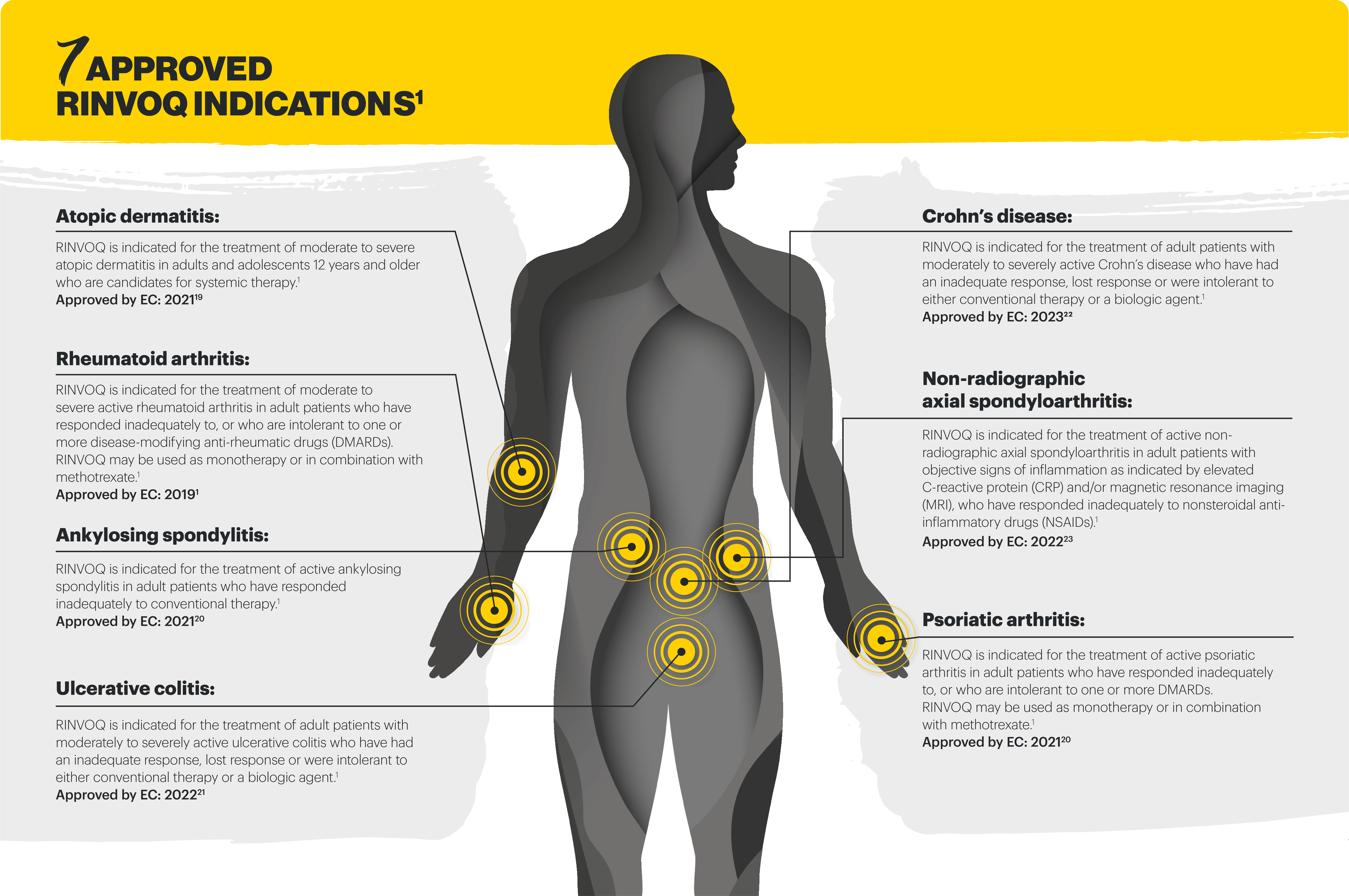
RINVOQ® (upadacitinib) is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis (UC) who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.1
RINVOQ achieved the primary endpoints of clinical remission per adapted Mayo score at Induction Week 8 and Maintenance Week 521,2
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
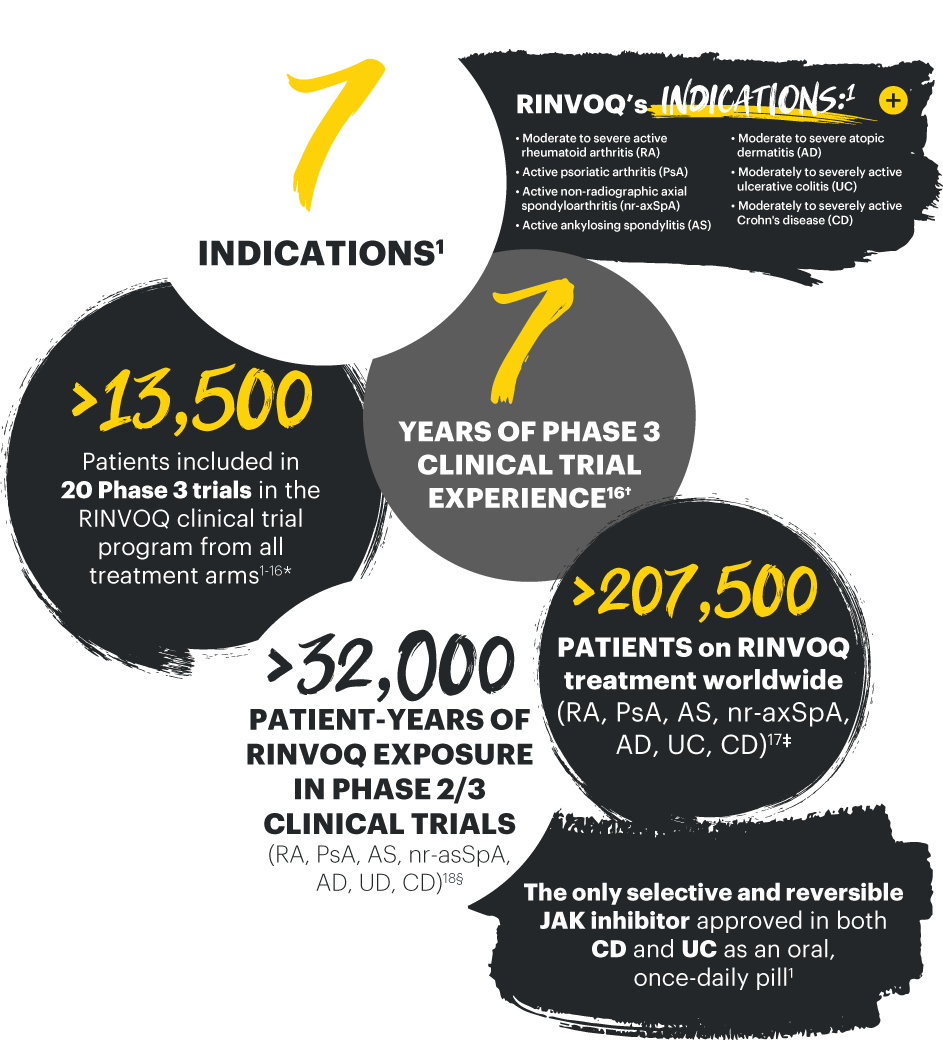
| * | Total patient number was calculated from adding the number of patients randomized in 20 trials. 13,557 patients includes all patients across all arms (active treatment and placebo) in the following Phase 3 trials in RA (SELECT-EARLY, SELECT-NEXT, SELECT-COMPARE, SELECT-MONOTHERAPY, SELECT-BEYOND, SELECT-CHOICE), PsA (SELECT-PsA 1, SELECT-PsA 2), AS (SELECT-AXIS 1, SELECT-AXIS 2), nr-axSpA (SELECT-AXIS 2), AD (MEASURE UP 1, MEASURE UP 2, AD UP, HEADS UP), UC (U-ACHIEVE [UC-1 and UC-3], U-ACCOMPLISH [UC-2]), and CD (U-EXCEED [CD-1], U-EXCEL [CD-2], and U-ENDURE [CD-3]). 7,932 |
| † | Beginning in RA. First patients dosed December 2015 in an RA Phase 3 clinical trial. |
| ‡ | 124,829 RA patients, 34,900 axSpA and PsA patients, 36,808 AD patients, 11,180 UC patients, and 13 CD patients taking RINVOQ globally. Global patient numbers include OUS+US through March 2023. Numbers are subject to change based on local market data adjustments. |
| § | 32,656 patient-years exposure. |
*Non-registrational clinical trial. †Phase 2b/3 clinical trial.
NOTE TO AFFILIATES:
Please evaluate use of ALL claims, graphs/ tables, and corresponding references (e.g., Data on File, abstracts, posters, manuscripts) according to local standards, codes, and regulations.
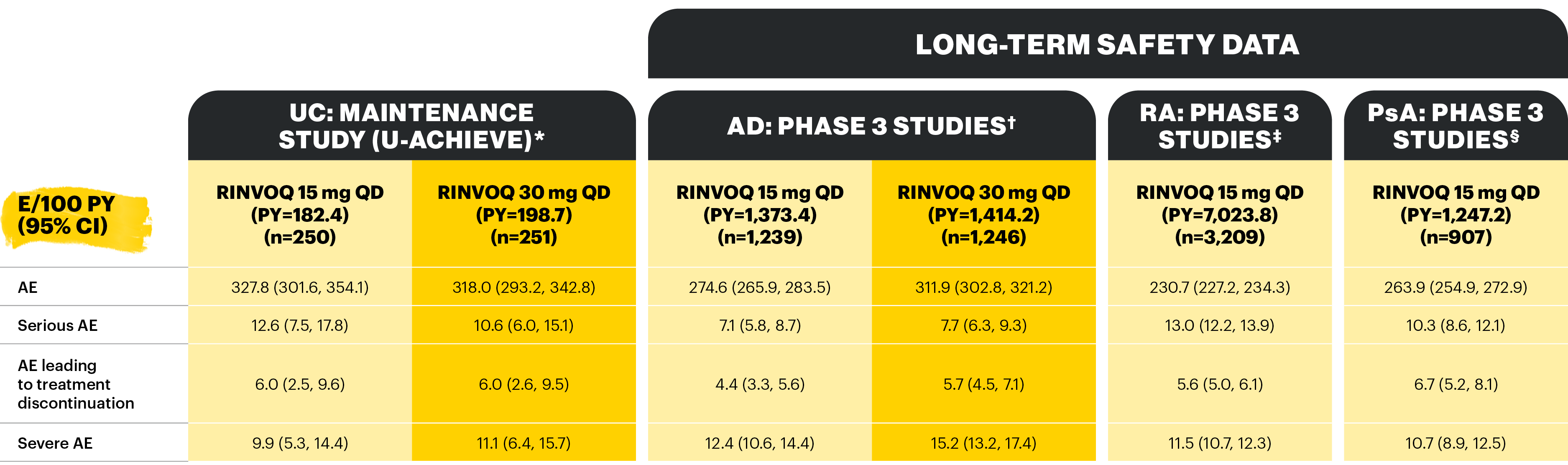
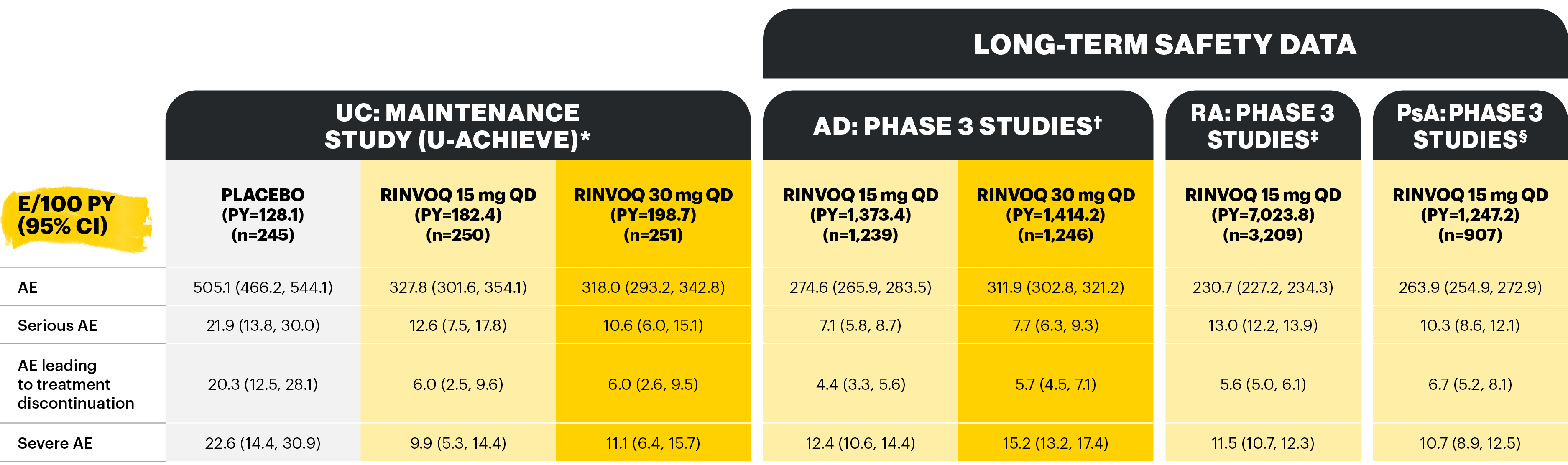
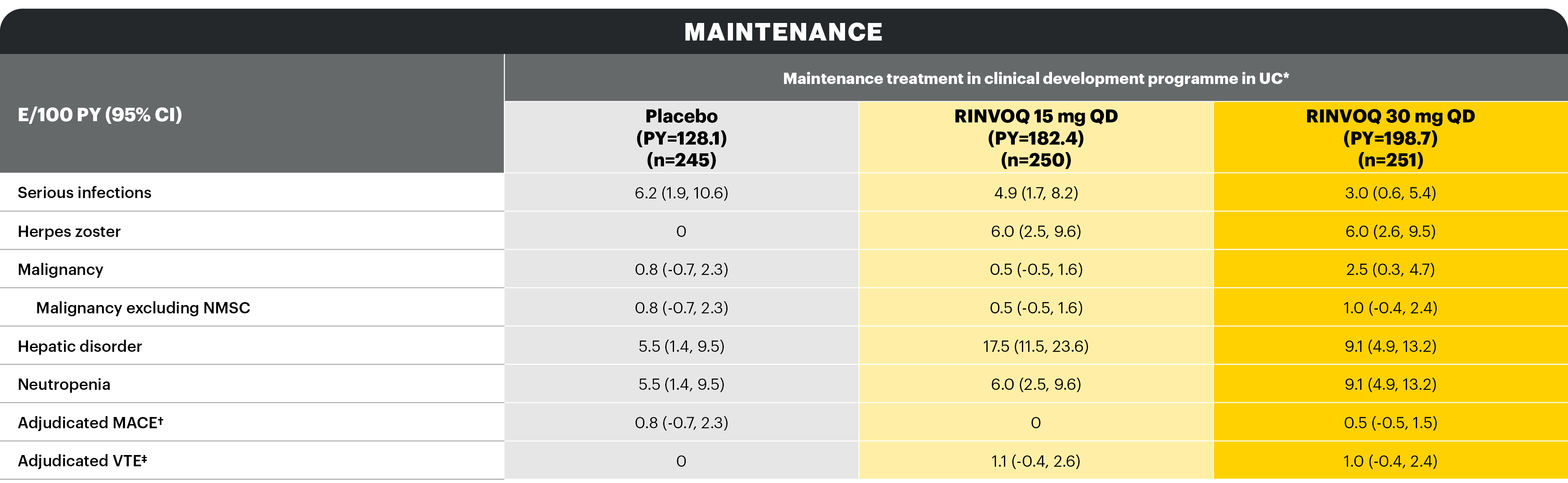
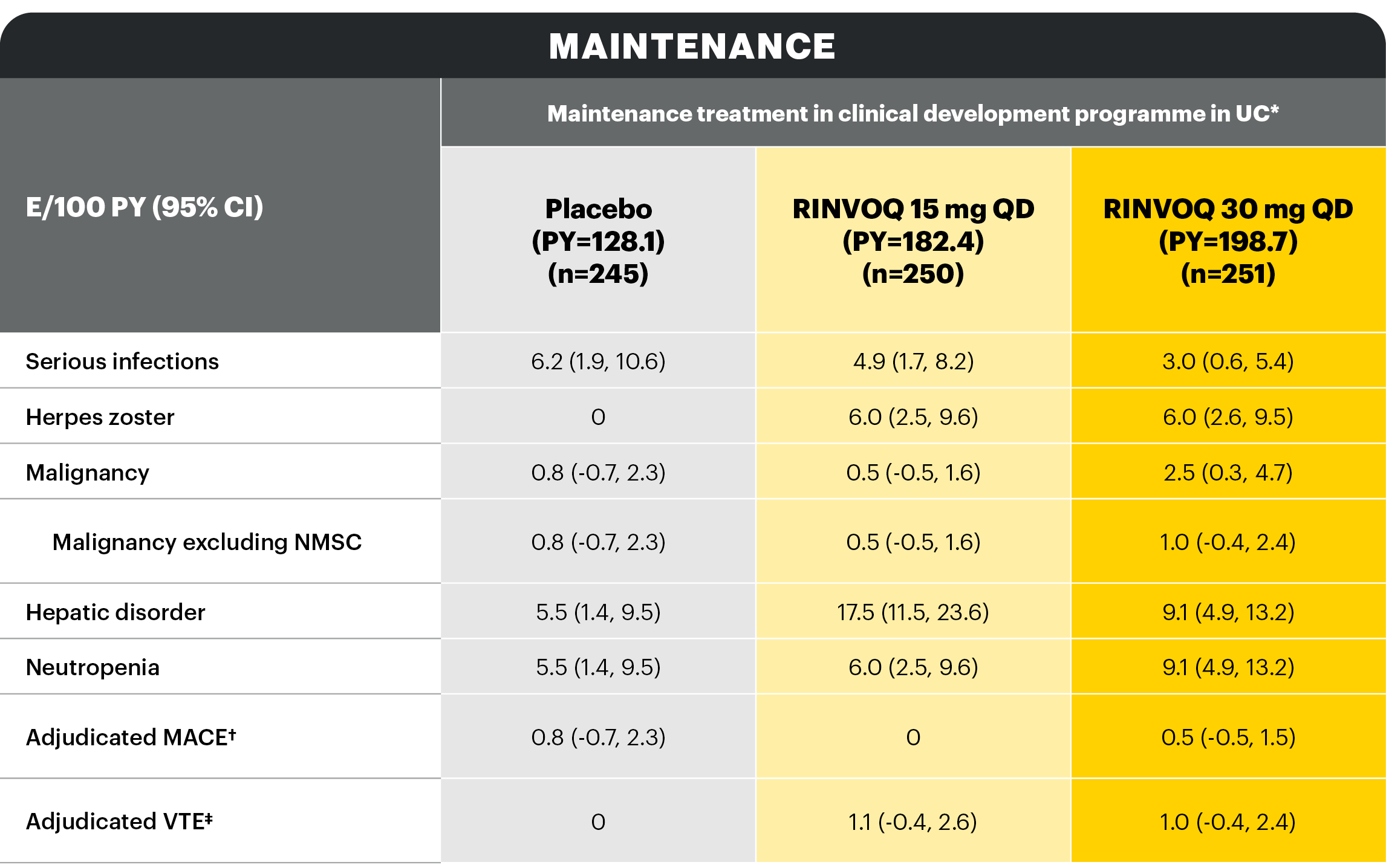
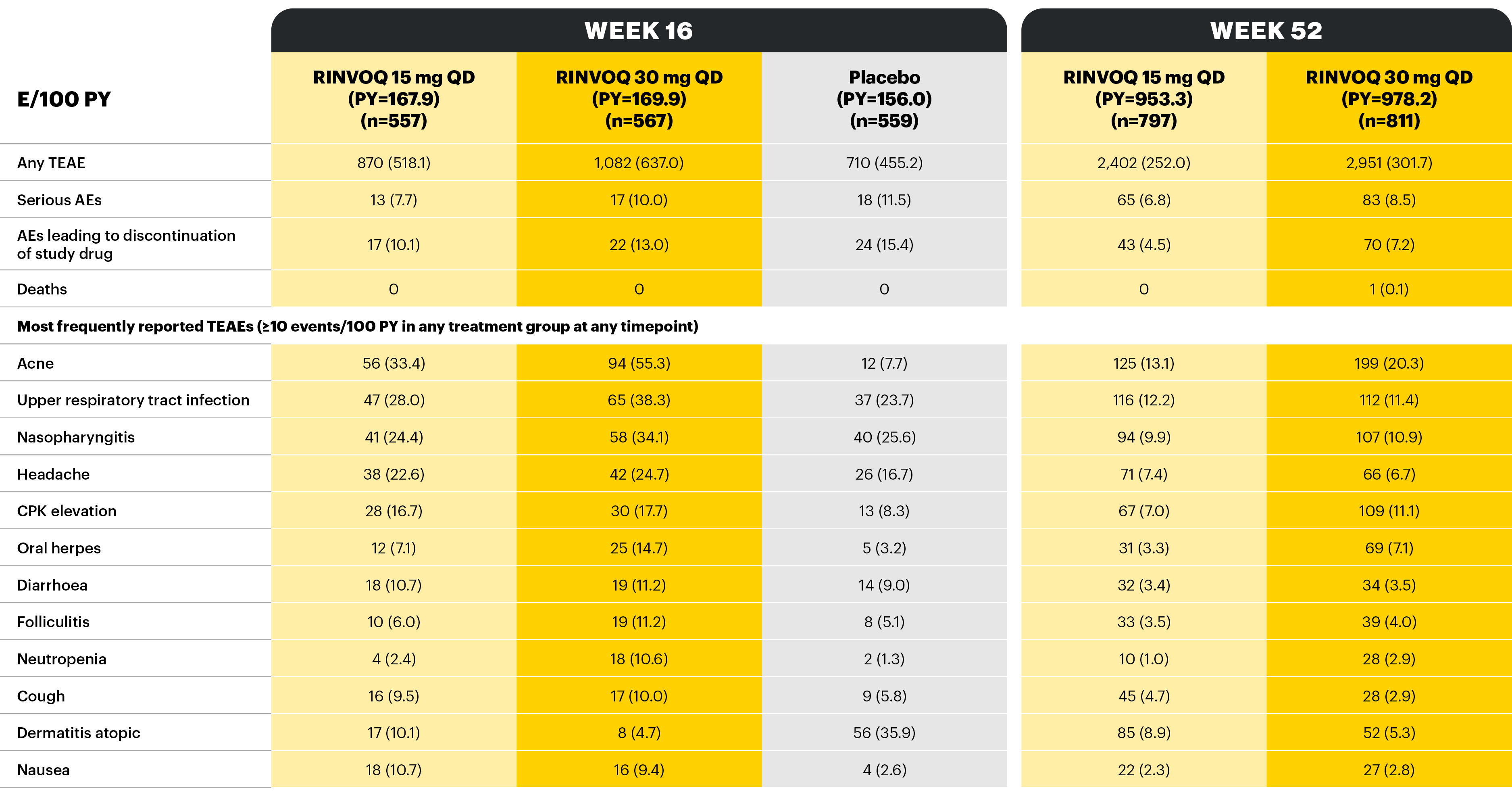
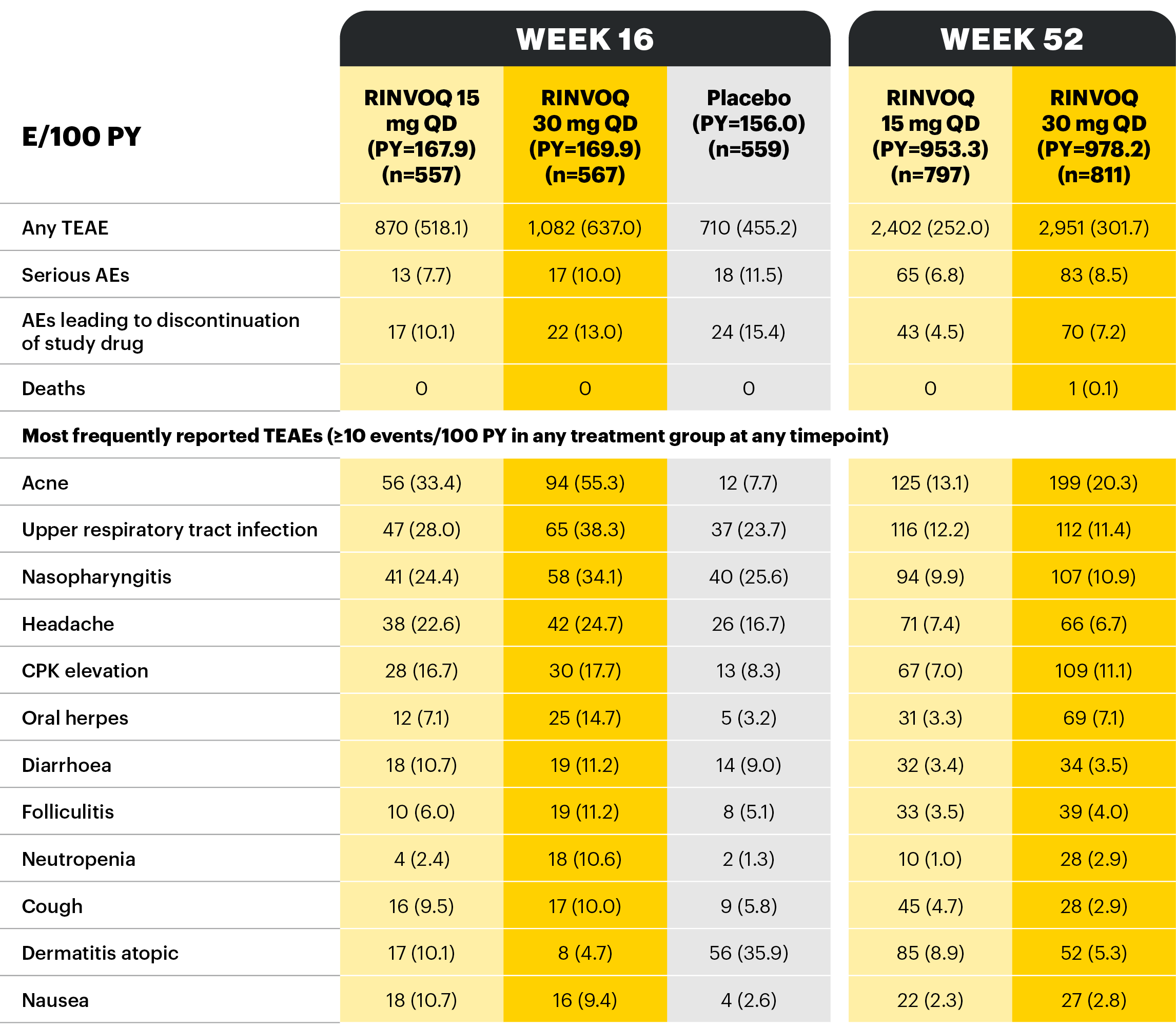
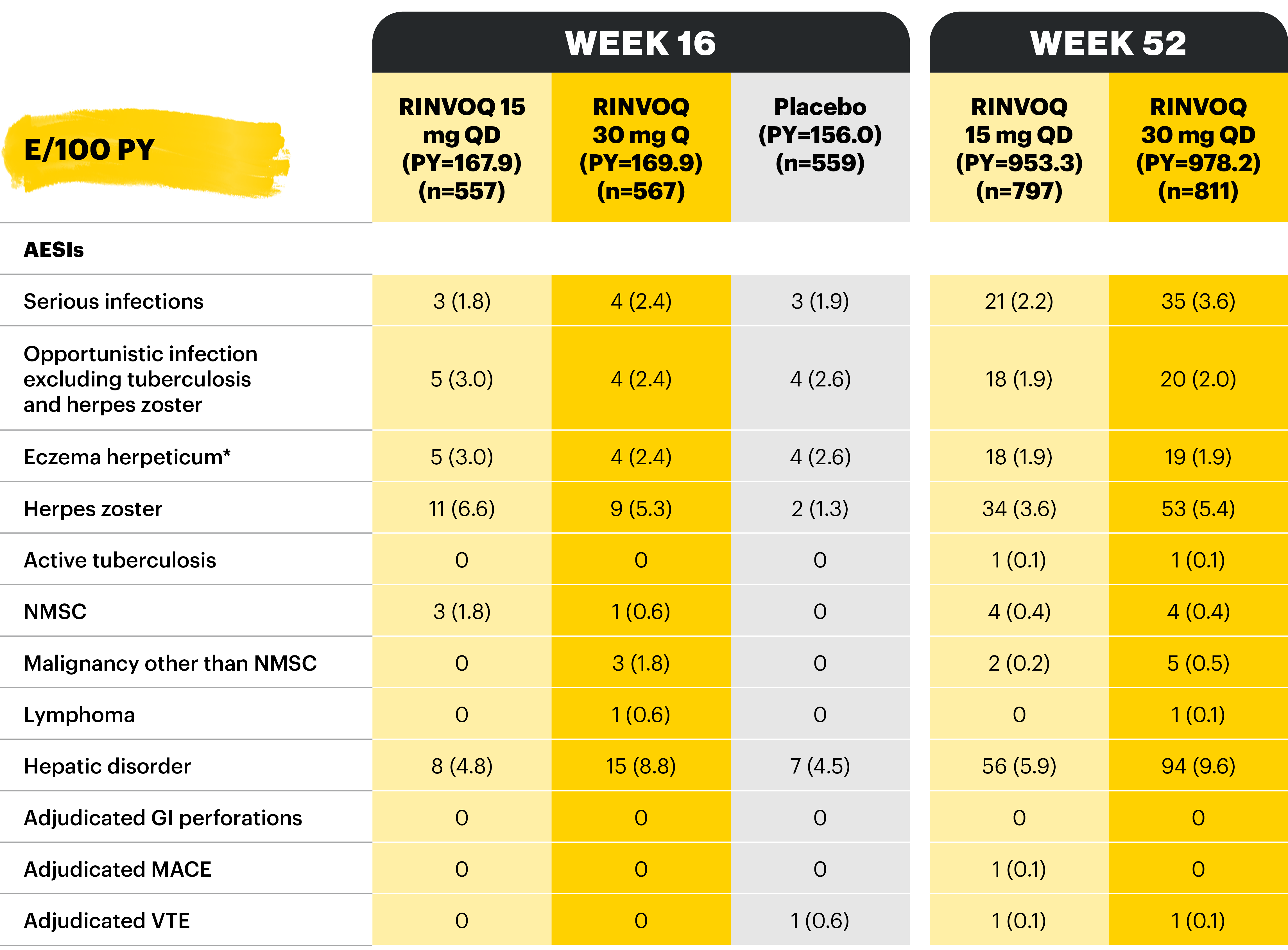
Overview of AE rates per 100 PYs with RINVOQ 15 mg QD and 30 mg QD
Objective: This assessment reviewed safety data for RINVOQ maintenance therapy in UC in the context of the known long-term safety profile of RINVOQ in its approved indications.
Table adapted from Colombel et al 2022; not all treatment arms are shown here.
*Patients who were RINVOQ 45 mg QD 8-week induction responders and were enrolled into maintenance treatment for 44 or 52 weeks. †Includes two Phase 3 studies; includes adults and adolescents. ‡Includes six Phase 3 studies; may include patients receiving concomitant methotrexate. §Includes two Phase 3 studies.
With RINVOQ maintenance treatment in UC through 52 weeks
Table adapted from Colombel et al 2022.
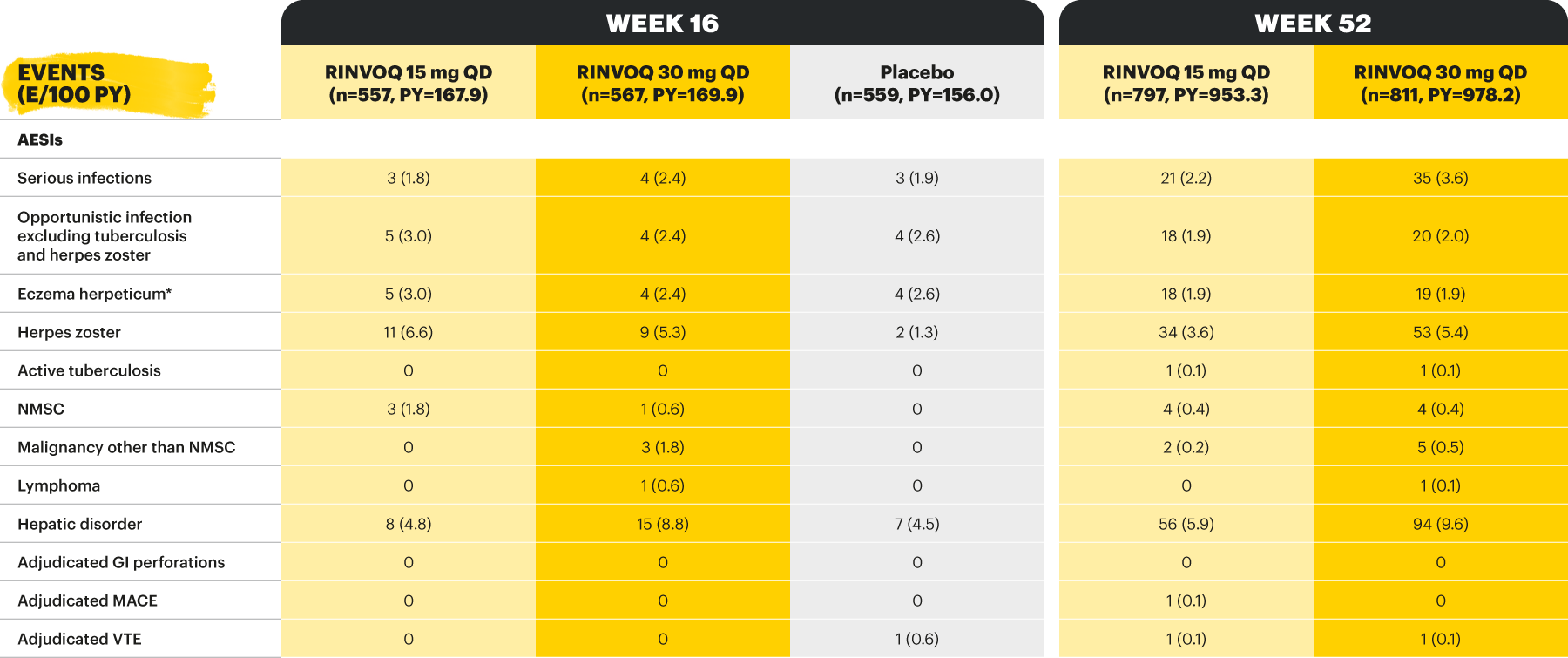
AEs of special interest were pre-specified based on previous studies with RINVOQ or other JAK inhibitors, e.g., serious infection, herpes zoster, malignancy, MACEs, and VTEs. MACEs and VTEs were adjudicated by an independent external adjudication committee.
*Patients who were RINVOQ 45 mg QD 8-week induction responders and were enrolled under the protocol for 44 or 52 weeks’ maintenance treatment. †Defined as cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke. ‡Defined as deep vein thrombosis and pulmonary embolism (fatal and non-fatal).
Potential cardiovascular and arterial and venous thromboembolic events were adjudicated by a masked, independent Cardiovascular Adjudication Committee. GI perforations were blindly adjudicated by sponsor employed experts.11
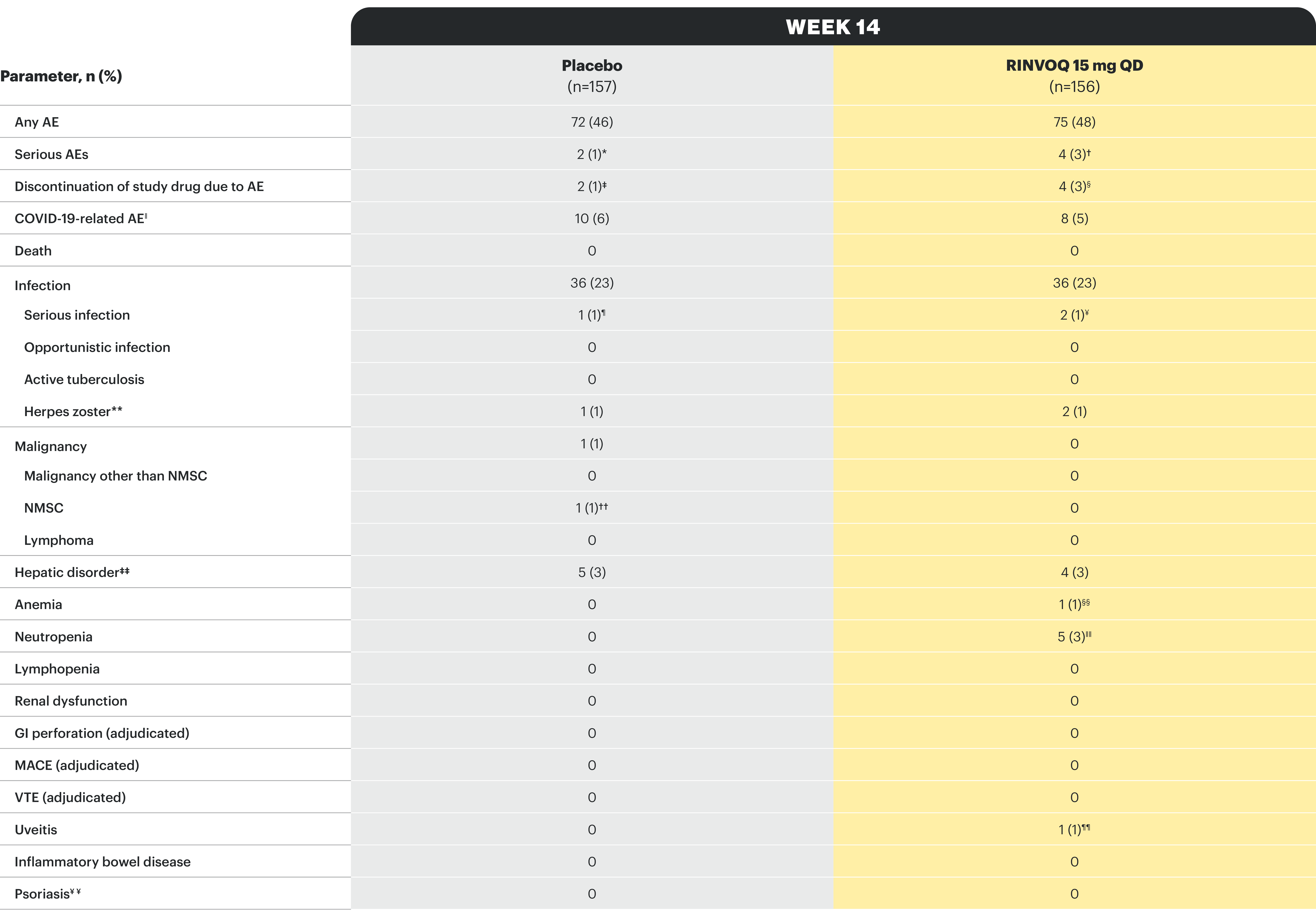
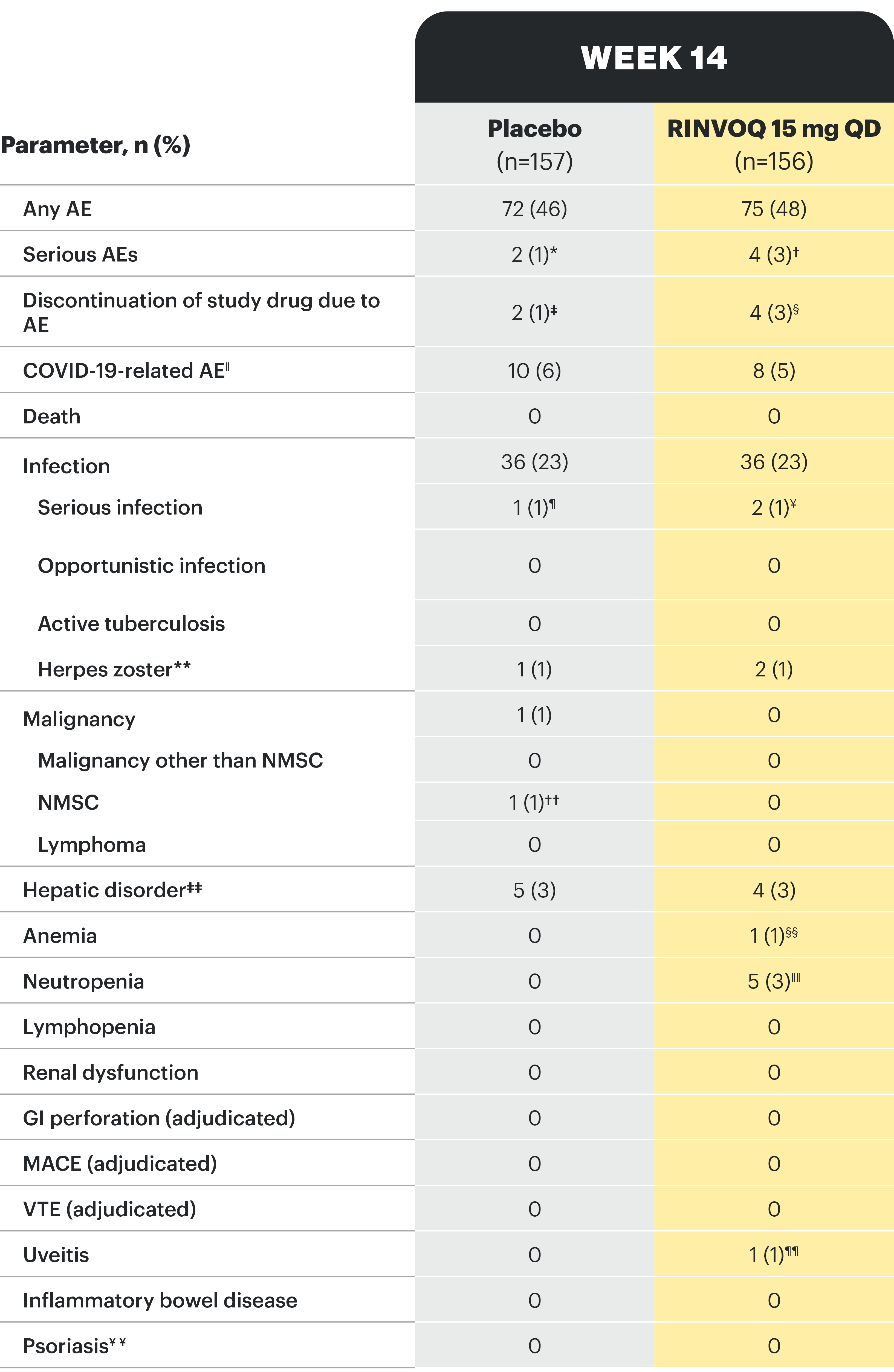
*One patient each with hemorrhagic fever with renal syndrome and pancreatitis. †One patient each with COVID-19 pneumonia, pyelonephritis, foot fracture, and knee osteoarthritis. ‡One patient each with moderate axial spondyloarthritis and mild vomiting. §Two patients with moderate axial spondyloarthritis, one patient with severe rash, moderate headache, and mild tremor, and one patient with mild abdominal pain and nausea. ǁBased on investigator assessment of adverse events associated with COVID-19 and not limited to preferred terms of COVID-19. ¶One patient with hemorrhagic fever with renal syndrome. ¥One patient each with COVID-19 pneumonia and pyelonephritis. **All herpes zoster events were non-serious and mild or moderate, and limited to one dermatome; both events in the RINVOQ group resolved without study drug interruption. ††One patient with basal cell carcinoma. ‡‡All events of hepatic disorder were non-serious and mild or moderate aminotransferase elevations; one event led to interruption of study drug; none led to study drug discontinuation. §§Event of anemia was nonserious, transient, and did not lead to study drug discontinuation. ǁǁAll neutropenia events were non-serious: 4 were mild or moderate in severity, and one was severe; the event of severe neutropenia occurred at baseline and resolved before study drug initiation. ¶¶Event occurred in a patient with a history of uveitis. ¥¥AE of psoriasis was based on 12 psoriasis-related preferred terms, including psoriasis.
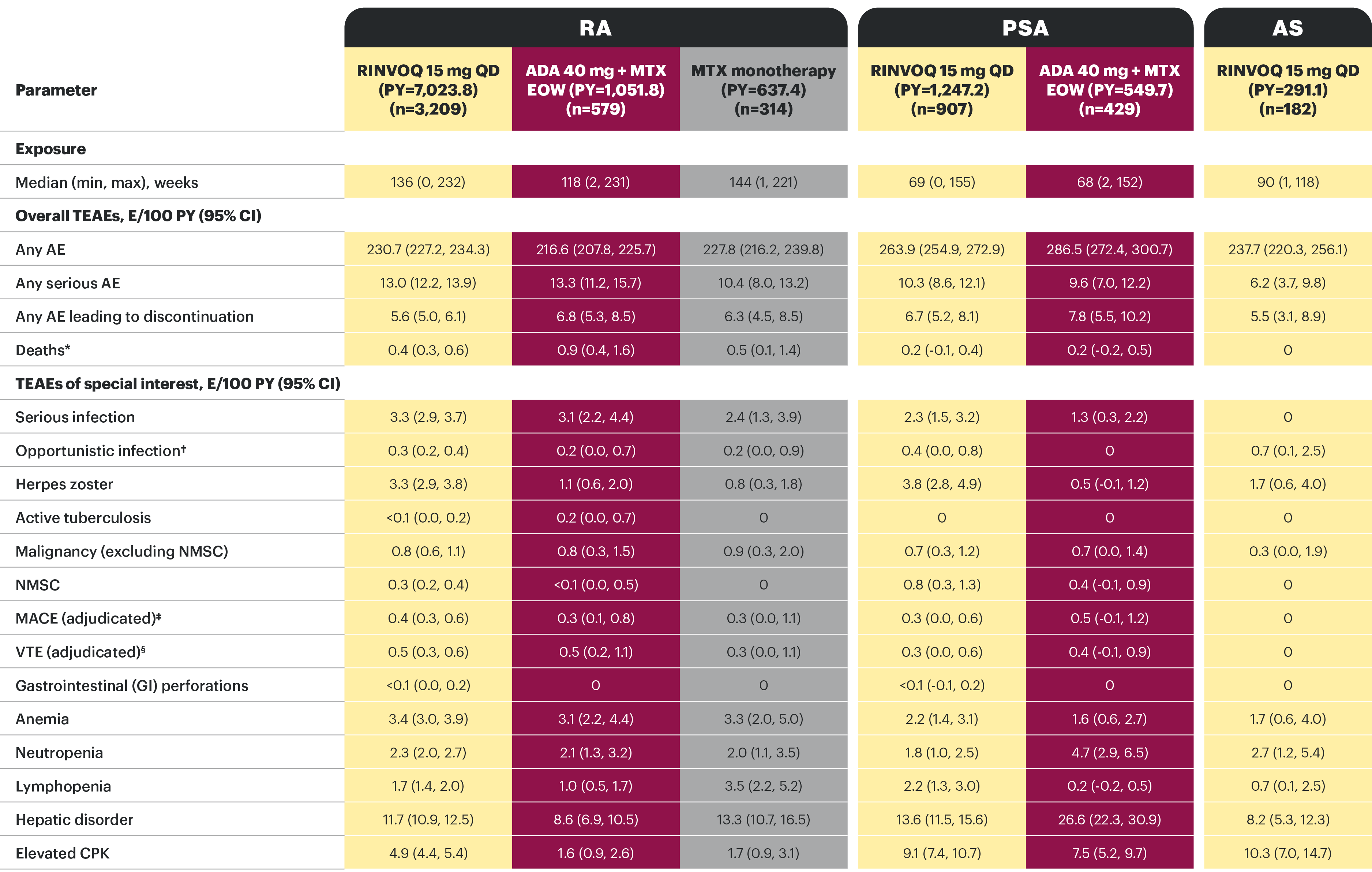
TEAEs in patients with moderate to severe active RA, active PsA, or active AS treated with RINVOQ, ADA, or MTX across RA, PsA, and AS trials25
RINVOQ data to date in both RA and PsA do not suggest that the observed rates of malignancy (excluding NMSC), MACE or VTE are elevated over those observed with ADA, or that the rate of mortality is above that expected in the general population.25 With the exception of herpes zoster and CPK elevations, EAERs were generally similar across RINVOQ, ADA, and MTX in RA, as well as RINVOQ and ADA in PsA. No new safety risks were identified with long-term treatment.25
*Includes non-treatment emergent. Rates of death reported in these clinical studies were not higher than expected in the general populations (standardized mortality ratio [95% CI]: 0.51 [0.39, 0.66] and 0.14 [0.03, 0.42] in RA and PsA, respectively; no deaths occurred in AS). †Excluding tuberculosis and herpes zoster. ‡MACE defined as cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke. §VTE includes DVT and PE.
PsA: A higher rate of serious infections (2.6 and 1.3 E/100 PYs, respectively) and hepatic transaminase elevations (ALT elevations Grade 3 and higher rates 1.4% and 0.4%, respectively) was observed in patients treated with RINVOQ + MTX therapy vs patients treated with monotherapy.1
Deaths, cardiovascular events, VTEs, and GI perforations were adjudicated by blinded independent committees using pre-specified definitions.
Treatment-emergent adverse events and exposure-adjusted event rates (events/100 PY) during RINVOQ administration (safety population, combined data, MEASURE UP 1 & 2)
At Week 16, patients receiving RINVOQ 15 mg QD or 30 mg QD during the double-blind period continued their assigned treatment in the blinded extension period, whereas patients receiving placebo were rerandomized 1:1 to receive RINVOQ 15 mg QD or 30 mg QD in the blinded extension period.
*Eczema herpeticum or Kaposi’s varicelliform eruption.
AD: atopic dermatitis; ADA: adalimumab; AE: adverse events; AESI: adverse event of special interest; ALT: alanine transaminase; aMs: adapted Mayo score; AS: ankylosing spondylitis; bDMARD: biologic disease-modifying antirheumatic drug; CD: Crohn's disease; COVID-19: coronavirus disease 2019; CPK: creatine phosphokinase; DVT: deep vein thrombosis; EAER: exposure-adjusted adverse event rate; EC: European Commission; ESS: endoscopic subscore; EOW; every other week; GI: gastrointestinal; IR: intolerance or inadequate response; JAK: Janus kinase; MACE: major adverse cardiac event; MTX: methotrexate; NMSC: nonmelanoma skin cancer; nr-axSpA: non-radiographic axial spondyloarthritis; OUS: outside the United States; PE: pulmonary embolism; PsA: psoriatic arthritis; PY: patient-years; QD: once daily; RA: rheumatoid arthritis; RBS: rectal bleeding score; TEAE: treatment-emergent adverse event; UC: ulcerative colitis; VTE: venous thromboembolism.
Study designs: U-ACHIEVE Induction (UC-1) and U-ACCOMPLISH (UC-2) were replicate induction studies, both of which were multicenter, double-blind, placebo-controlled clinical studies. In UC-1 and UC-2, 988 patients (473 and 515 patients, respectively) were randomized to RINVOQ 45 mg QD or placebo for 8 weeks with a 2:1 treatment allocation ratio and included in the efficacy analysis. All enrolled patients had moderately to severely active UC defined as aMs of 5 to 9 with an ESS of 2 or 3 and demonstrated prior treatment failure including inadequate response, loss of response, or intolerance to prior conventional and/or biologic treatment. U-ACHIEVE Maintenance (UC-3) was a multicenter, double-blind, placebo-controlled clinical study with 451 patients who achieved clinical response per aMs (decrease ≥2 points and ≥30% from baseline and a decrease in RBS ≥1 from baseline or an absolute RBS ≤1) with 8-week RINVOQ 45 mg QD induction treatment. Patients were rerandomized 1:1:1 to receive either RINVOQ 15 mg QD, 30 mg QD, or placebo.1,2
UP NEXT
RINVOQ is an oral, once daily, selective and reversible JAK inhibitor now approved for the treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.1
RINVOQ can be taken at any time of the day, with or without food. Tablets should be swallowed whole and should not be split, crushed, or chewed in order to ensure the entire dose is delivered correctly.1
A Phase 3 clinical trial program involving 3 studies: 2 replicate induction studies and 1 maintenance study evaluated RINVOQ 45 mg QD vs placebo for induction and RINVOQ 15 mg QD and 30 mg QD vs placebo for maintenance treatment.1,2
RINVOQ ISI PLACEHOLDER
and
HUMIRA ISI PLACEHOLDER and link to HUMIRA SmPC
[Please insert local summary of safety]
REFERENCES
- RINVOQ [Summary of Product Characteristics]. AbbVie Deutschland GmbH & Co. KG; December 2023.
- Danese S, Vermeire S, Zhou W, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399(10341):2113-2128. doi:10.1016/S0140-6736(22)00581-5
- Rubbert-Roth A, Enejosa J, Pangan AL, et al. Trial of upadacitinib or abatacept in rheumatoid arthritis. N Engl J Med. 2020;383(16):1511-1521. doi:10.1056/NEJMoa2008250
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157(9):1047-1055. doi:10.1001/jamadermatol.2021.3023
- van Vollenhoven R, Takeuchi T, Pangan AL, et al. Efficacy and safety of upadacitinib monotherapy in methotrexate-naive patients with moderately-to-severely active rheumatoid arthritis (SELECT-EARLY): a multicenter, multi-country, randomized, double-blind, active comparator-controlled trial. Arthritis Rheumatol. 2020;72(10):1607-1620.doi:10.1002/art.41384
- Fleischmann R, Pangan AL, Song IH, et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind, randomized controlled trial. Arthritis Rheumatol. 2019;71(11):1788-1800. doi:10.1002/art.41032
- Smolen JS, Pangan AL, Emery P, et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): a randomised, placebo-controlled, double-blind phase 3 study. Lancet. 2019;393(10188):2303-2311. doi:10.1016/S0140-6736(19)30419-2
- Genovese MC, Fleischmann R, Combe B, et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet. 2018;391(10139):2513-2524. doi:10.1016/S0140-6736(18)31116-4
- McInnes IB, Anderson JK, Magrey M, et al. Trial of upadacitinib and adalimumab for psoriatic arthritis. N Engl J Med. 2021;384(13):1227-1239. doi:10.1056/NEJMoa2022516
- Mease PJ, Lertratanakul A, Anderson JK, et al. Upadacitinib for psoriatic arthritis refractory to biologics: SELECT-PsA 2. Ann Rheum Dis. 2021;80(3):312-320. doi:10.1136/annrheumdis-2020-218870
- Deodhar A, Van den Bosch F, Poddubnyy D, et al. Upadacitinib for the treatment of active non-radiographic axial spondyloarthritis (SELECT-AXIS 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2022;400(10349):369-379. doi:10.1016/S0140-6736(22)01212-0
- van der Heijde D, Song IH, Pangan AL, et al. Efficacy and safety of upadacitinib in patients with active ankylosing spondylitis (SELECT-AXIS 1): a multicentre, randomised, double-blind, placebo-controlled, phase 2/3 trial. Lancet. 2019;394(10214):2108-2117. doi:10.1016/S0140-6736(19)32534-6
- van der Heijde D, Baraliakos X, Sieper J, et al. Efficacy and safety of upadacitinib for active ankylosing spondylitis refractory to biological therapy: a double-blind, randomised, placebo-controlled phase 3 trial. Ann Rheum Dis. 2022;81(11):1515-1523. doi:10.1136/ard-2022-222608
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials Lancet. 2021;397(10290):2151-2168. doi:10.1016/S0140-6736(21)00588-2
- Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397(10290):2169-2181. doi:10.1016/S0140-6736(21)00589-4
- Burmester GR, Kremer JM, Van den Bosch F, et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10139):2503-2512. doi:10.1016/S0140-6736(18)31115-2
- Data on file, AbbVie Inc. ABVRRTI76444.
- Data on file, AbbVie Inc. ABVRRTI75395.
- European Commission approves RINVOQ® (upadacitinib) as first JAK inhibitor in the European Union for the treatment of both adults and adolescents with moderate to severe atopic dermatitis. News release. AbbVie. August 24, 2021. Accessed December 6, 2023. https://news.abbvie.com/2021-08-24-European-Commission-Approves-RINVOQ-R-upadacitinib-as-First-JAK-Inhibitor-in-the-European-Union-for-the-Treatment-of-Both-Adults-and-Adolescents-with-Moderate-to-Severe-Atopic-Dermatitis
- European Commission approves AbbVie's RINVOQ™ (upadacitinib) for the treatment of psoriatic arthritis and ankylosing spondylitis. News release. AbbVie. January 25, 2021. Accessed December 6, 2023. https://news.abbvie.com/2021-01-25-European-Commission-Approves-AbbVies-RINVOQ-TM-Upadacitinib-for-the-Treatment-of-Psoriatic-Arthritis-and-Ankylosing-Spondylitis
- European Commission approves RINVOQ® (upadacitinib) for the treatment of adults with moderate to severe ulcerative colitis. News release. AbbVie. July 26, 2022. Accessed December 6, 2023. https://news.abbvie.com/2022-07-26-European-Commission-Approves-RINVOQ-R-upadacitinib-for-the-Treatment-of-Adults-With-Moderate-to-Severe-Ulcerative-Colitis
- AbbVie announces European Commission Approval of RINVOQ® (upadacitinib) for the treatment of moderately to severely active Crohn's disease. News release. AbbVie. April 17, 2023. Accessed December 6, 2023. https://news.abbvie.com/2023-04-17-AbbVie-Announces-European-Commission-Approval-of-RINVOQ-R-upadacitinib-for-the-Treatment-of-Moderately-to-Severely-Active-Crohns-Disease
- RINVOQ® (upadacitinib) approved by European Commission as an oral treatment for adults with active non-radiographic axial spondyloarthritis. News release. AbbVie. July 29, 2022. Accessed December 6, 2023. https://news.abbvie.com/2022-07-29-RINVOQ-R-upadacitinib-Approved-by-European-Commission-as-an-Oral-Treatment-for-Adults-with-Active-Non-Radiographic-Axial-Spondyloarthritis
- Colombel JF, Panaccione R, Nakase H, et al. The safety profile of upadacitinib maintenance therapy in ulcerative colitis in the phase 3 U-ACHIEVE study is consistent with that in approved indications. J Crohns Colitis. 2022;16(suppl 1):i514. doi:10.1093/ecco-jcc/jjab232.699
- Burmester G, Cohen S, Winthrop K, et al. Long-term safety profile of upadacitinib in patients with rheumatoid arthritis, psoriatic arthritis, or anklosing spondylitis. Poster presented at: American College of Rheumatology (ACR) Virtual Convergence; November 5-9, 2021.
- Simpson EL, Papp KA, Blauvelt A, et al. Efficacy and safety of upadacitinib in patients with atopic dermatitis: results through week 52 from replicate, phase 3, randomized, double-blind, placebo-controlled studies: Measure Up 1 and Measure Up 2. Poster presented at: Dermatology Education Foundation (DEF) Essential Resource Meeting (DERM2021); August 5-8, 2021; Las Vegas, NV.