RINVOQ® (upadacitinib) is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis (UC) who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.1
RINVOQ achieved the primary endpoints of clinical remission per adapted Mayo score at Induction Week 8 and Maintenance Week 521,2
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
A Phase 3 trial program involving 3 studies: 2 replicate induction studies (U-ACHIEVE Induction and U-ACCOMPLISH) and 1 maintenance study (U-ACHIEVE Maintenance). A total of 988 patients with moderately to severely active UC evaluating RINVOQ 45 mg QD vs placebo for induction and RINVOQ 15 mg QD and 30 mg QD vs placebo for maintenance treatment (N=451).1*
*Patients who achieved clinical response per adapted Mayo score with 8-week RINVOQ 45 mg QD induction treatment entered maintenance.
This post hoc analysis evaluated the comparative benefit-risk profile of RINVOQ vs placebo as induction and maintenance therapy in patients with moderately to severely active ulcerative colitis.3*
The RINVOQ UC program enrolled patients with active disease, inadequate response, loss of response, or intolerance to ≥1 oral aminosalicylate, corticosteroid, immunosuppressant, and/or biologic therapy to 3 induction trials (U-ACHIEVE [one Phase 2b and one Phase 3]), U-ACCOMPLISH (Phase 3), and the Phase 3 U-ACHIEVE maintenance study.3
Limitations of this post hoc analysis:
- Restriction to an 8-week induction and 52-week maintenance therapeutic regimen with limited patient exposure might limit detection and interpretation of adverse events with low incidences (e.g., malignancy).2
- Lack of dose adjustment during maintenance treatment (e.g., patients could not increase to 30 mg if the 15 mg dose was ineffective).2
Post-hoc analyses should not be used for individual prescribing decisions or dose comparison. The RINVOQ Summary of Product Characteristics should be consulted and a benefit-risk assessment should be conducted for individual patients before starting RINVOQ therapy.
AbbVie funded this trial and participated in the trial design, research, analysis, data collection, interpretation of data, and the review and approval of the publication.
*Results are based on nonresponder imputation incorporating multiple imputation to handle missing data due to COVID-19. The point estimate and 95% CI for treatment difference are based on Cochran-Mantel-Haenszel tests adjusted for strata (induction: stratified by corticosteroid use at baseline [yes vs no], adapted Mayo score at baseline [≤7 vs >7], and bio-IR status [bio-IR vs non-bio-IR]; maintenance: stratified by bio-IR status [bio-IR vs non-bio-IR], corticosteroid use at Week 0 of maintenance [yes vs no], and clinical remission status at Week 0 of maintenance [yes vs no]).
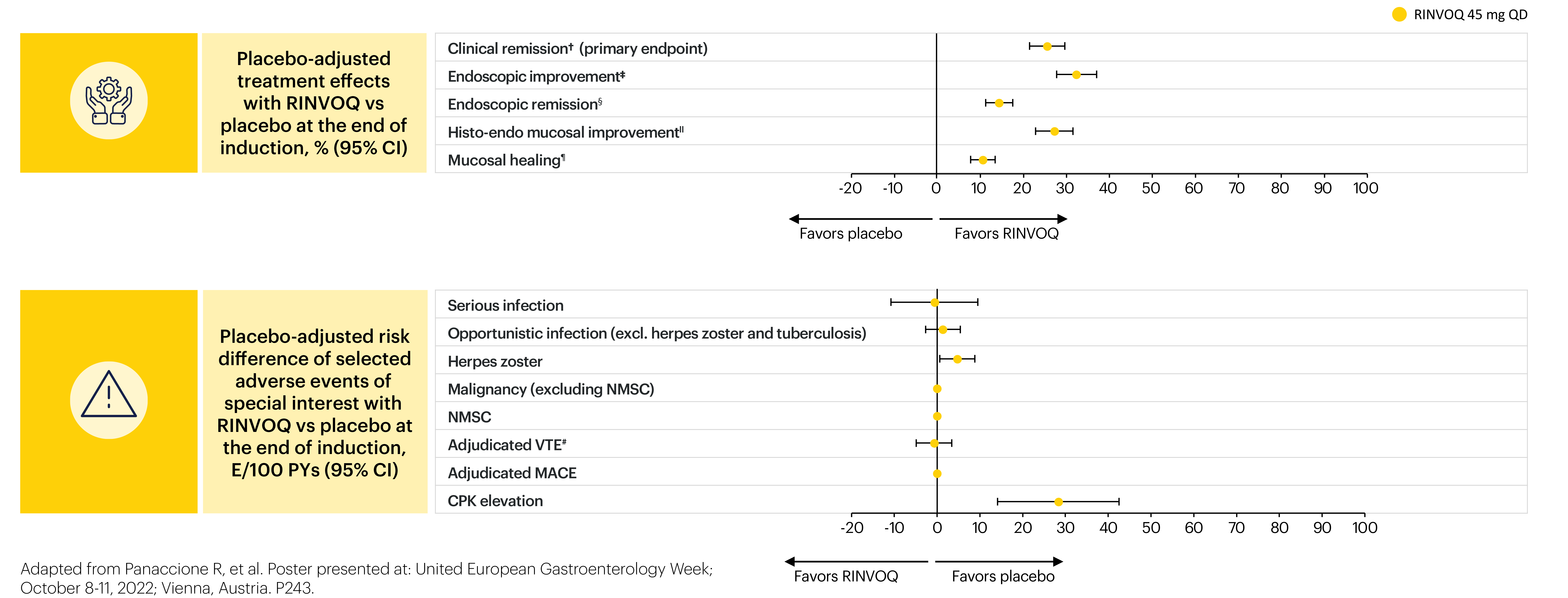
A positive value indicates more favorable efficacy with RINVOQ vs placebo; a negative value indicates more favorable safety with RINVOQ vs placebo.
*Patients were inducted onto therapy either in the placebo group (n=328) or RINVOQ 45 mg QD group (n=660). The safety population included all patients who received ≥1 dose of study therapy (intent-to-treat population). †Adapted Mayo score ≤2, stool frequency subscore ≤1 and not greater than baseline, rectal bleeding subscore=0, and ESS ≤1 without friability. ‡ESS ≤1. §ESS=0. IIESS ≤1 without friability and Geboes score ≤3.1. ¶ESS=0 and Geboes score <2. #The placebo event occurred in the Phase 3 study and the RINVOQ 45 mg QD event in a Phase 2b induction study.
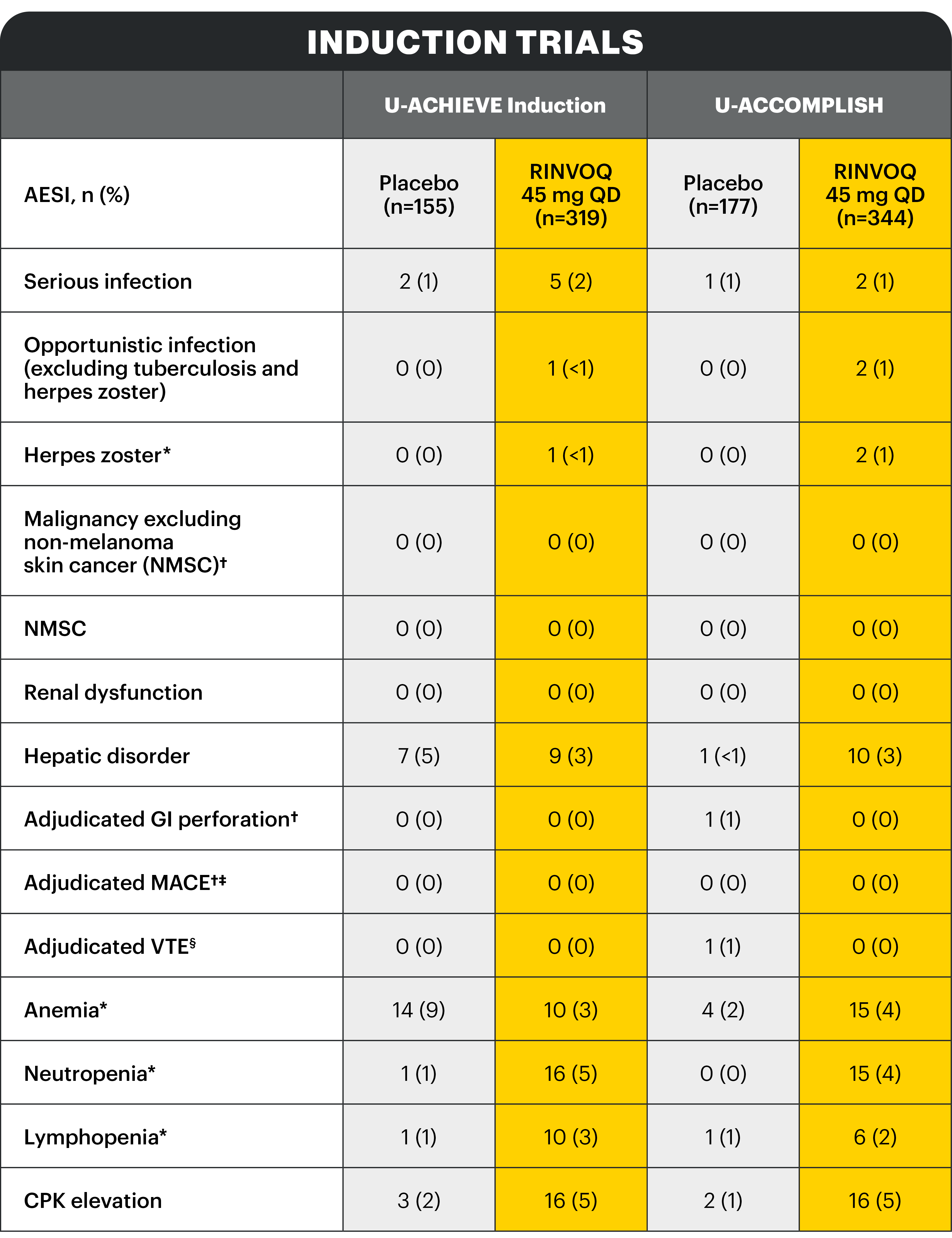
In the induction period, 27 (7.1%) patients discontinued treatment because of an adverse event (AE) with placebo, compared with 17 (2.4%) with RINVOQ 45 mg QD. In the maintenance period, 25 (10.2%), 10 (4.0%), and 18 (7.2%) patients discontinued treatment due to an AE with placebo, RINVOQ 15 mg QD, and RINVOQ 30 mg QD, respectively.
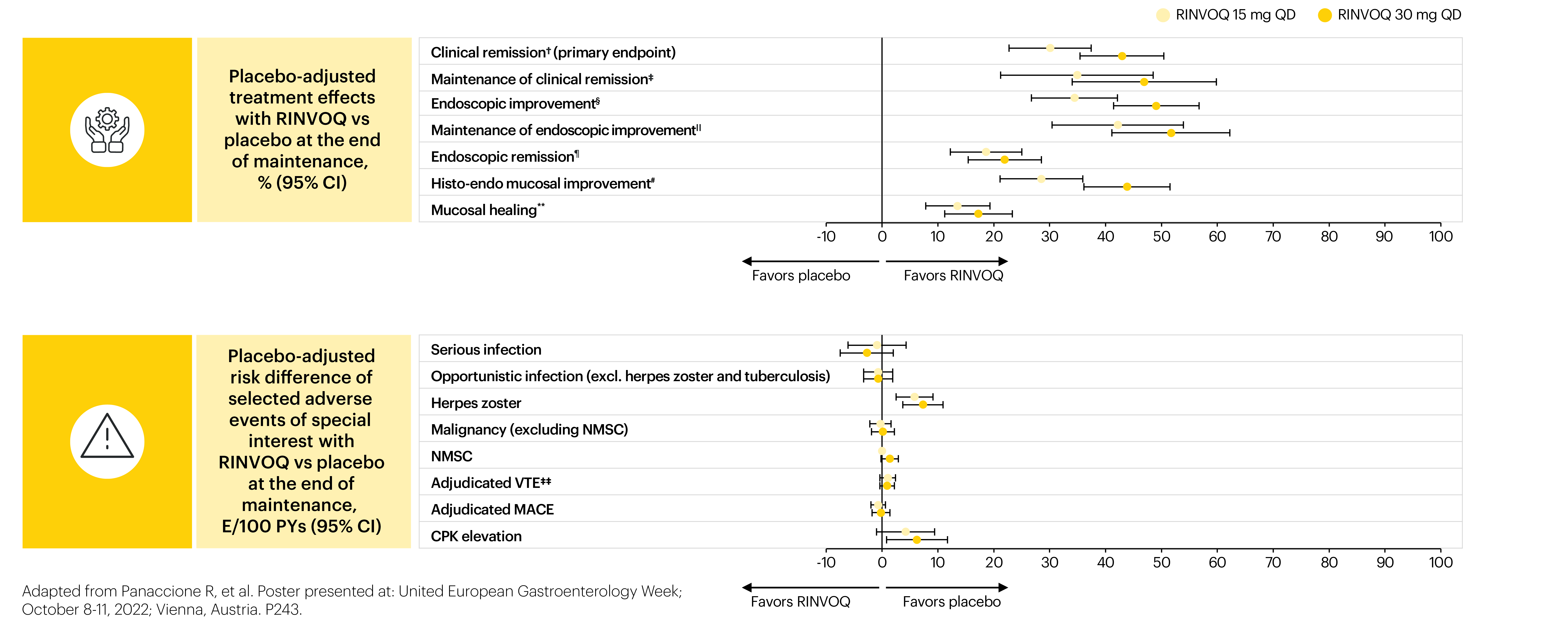
A positive value indicates more favorable efficacy with RINVOQ vs placebo; a negative value indicates more favorable safety with RINVOQ vs placebo.
*Patients who achieved a clinical response after 8 weeks of induction treatment (RINVOQ 45 mg QD) were rerandomized to RINVOQ 15 mg or 30 mg QD or placebo for 52 weeks. 681 patients progressed onto maintenance therapy and were analyzed for efficacy (placebo, n=223; RINVOQ 15 mg, n=225; and RINVOQ 30 mg, n=233), and 746 for safety (n=245, n=250, and n=251, respectively). The safety population included all patients who received ≥1 dose of study therapy (intent-to-treat population plus patients who received up to 44 weeks of maintenance therapy under earlier versions of protocol amendments). †Adapted Mayo score ≤2, stool frequency subscore ≤1 and not greater than baseline, rectal bleeding subscore=0, and ESS ≤1 without friability. ‡Among patients with clinical remission at the end of the induction therapy. §ESS ≤1. IIAmong patients with endoscopic improvement at the end of the induction therapy. ¶ESS=0. #ESS ≤1 without friability and Geboes score ≤3.1. **ESS=0 and Geboes score <2. ‡‡The placebo event occurred in the Phase 3 study and the RINVOQ 45 mg QD event in a Phase 2b induction study.
For analysis of efficacy, point estimates and 95% confidence intervals of the placebo-adjusted treatment effect were calculated across the primary and key secondary endpoints in the intent-to-treat population.
For the safety risk analysis, the exposure-adjusted event rate (events/100 patient years) was calculated for selected adverse events of special interest (AESIs) in the initial induction phase with RINVOQ 45 mg QD and the maintenance phase with RINVOQ 15 mg QD and 30 mg QD.
- The placebo-adjusted risk was then calculated for each AESI.
Induction1
The recommended induction dose of RINVOQ is 45 mg once daily for 8 weeks. For patients who do not achieve adequate therapeutic benefit by Week 8, RINVOQ 45 mg once daily may be continued for an additional 8 weeks.*
Maintenance1
The recommended maintenance dose of RINVOQ is 15 mg or 30 mg once daily based on individual patient presentation:
- A dose of 15 mg is recommended for patients at higher risk of VTE, MACE, and malignancy.
- A dose of 30 mg once daily may be appropriate for some patients, such as those with high disease burden or requiring 16-week induction treatment who are not at higher risk of VTE, MACE, and malignancy or who do not show adequate therapeutic benefit to 15 mg once daily.
- The lowest effective dose to maintain response should be used.
For patients 65 years of age and older, the recommended dose is 15 mg once daily.
*A higher rate of herpes zoster was observed with an induction treatment period of 16 weeks vs 8 weeks.
RINVOQ should only be used if no suitable treatment alternatives are available in patients:1
- 65 years of age and older:
- patients with history of atherosclerotic cardiovascular disease or other cardiovascular risk factors (such as current or past long-time smokers);
- patients with malignancy risk factors (e.g., current malignancy or history of malignancy)
The long-term safety and efficacy of RINVOQ will continue to be evaluated.
Please refer to RINVOQ Important Safety Information and complete Summary of Product Characteristics for additional dosing information for patients on strong inhibitors of cytochrome P450 (CYP) 3A4, the elderly, and patients with renal or hepatic impairment.
Safety considerations1
Per SmPC across all RINVOQ indications
Herpes zoster
The risk of herpes zoster appears to be higher in Japanese patients treated with RINVOQ. If a patient develops herpes zoster, interruption of RINVOQ therapy should be considered until the episode resolves. Prior to initiating RINVOQ, it is recommended that patients be brought up to date with all immunizations, including prophylactic zoster vaccinations, in agreement with current immunization guidelines.
Serious infections
RINVOQ should not be initiated in patients with an active, serious infection, including localized infections. Treatment with RINVOQ therapy should be interrupted if a patient develops a serious or opportunistic infection.
A higher rate of serious infections was observed with RINVOQ 30 mg compared to RINVOQ 15 mg. As there is a higher incidence of infections in the elderly ≥65 years of age and in the diabetic population in general, caution should be used when treating the elderly and patients with diabetes. In patients 65 years of age and older, RINVOQ should only be used if no suitable treatment alternatives are available.
Lymphoma and other malignancies
Lymphoma and other malignancies have been reported in patients receiving JAK inhibitors, including RINVOQ. A higher rate of malignancies was observed with RINVOQ 30 mg compared to RINVOQ 15 mg.
In patients 65 years of age and older, patients who are current or past long-time smokers, or patients with other malignancy risk factors (e.g., current malignancy or history of malignancy), RINVOQ should only be used if no suitable treatment alternatives are available.
Non-melanoma skin cancer (NMSC)
NMSCs have been reported in patients treated with RINVOQ. A higher rate of NMSC was observed with RINVOQ 30 mg compared to RINVOQ 15 mg. Periodic skin examination is recommended for all patients, particularly those with risk factors for skin cancer.
Major adverse cardiac events (MACE)
Events of MACE were observed in clinical studies of RINVOQ. In patients 65 years of age and older, patients who are current or past long-time smokers, and patients with history of atherosclerotic cardiovascular disease or other cardiovascular risk factors, RINVOQ should only be used if no suitable treatment alternatives are available.
Deep vein thrombosis (DVT)
Events of deep venous thrombosis and pulmonary embolism (PE) were observed in clinical trials for RINVOQ. In patients with cardiovascular or malignancy risk factors, RINVOQ should only be used if no suitable treatment alternatives are available.
In patients with known VTE risk factors other than cardiovascular or malignancy risk factors, RINVOQ should be used with caution. VTE risk factors other than cardiovascular or malignancy risk factors include previous VTE patients undergoing major surgery, immobilization, use of combined hormonal contraceptives or hormone replacement therapy, and inherited coagulation disorder. Patients should be re-evaluated periodically during RINVOQ treatment to assess for changes in VTE risk. Promptly evaluate patients with signs and symptoms of VTE and discontinue RINVOQ in patients with suspected VTE, regardless of dose.
Please refer to RINVOQ Important Safety Information and complete Summary of Product Characteristics.
AE: adverse event; AESI: adverse event of special interest; rase; aMs: adapted Mayo score; bio-IR: biologic inadequate response; CI: confidence interval; CPK: creatine phosphokinase; CYP3A4: cytochrome P450 3A4; ESS: endoscopic subscore; IR: inadequate response; JAK: Janus kinase; MACE: major adverse cardiac event; NMSC: non-melanoma skin cancer; PY: patient-years; QD: once daily; RBS: rectal bleeding score; UC: ulcerative colitis; URTI: upper respiratory tract infection; VTE: venous thromboembolism.
Study designs: U-ACHIEVE Induction (UC-1) and U-ACCOMPLISH (UC-2) were replicate induction studies, both of which were multicenter, double-blind, placebo-controlled clinical studies. In UC-1 and UC-2, 988 patients (473 and 515 patients, respectively) were randomized to RINVOQ 45 mg QD or placebo for 8 weeks with a 2:1 treatment allocation ratio and included in the efficacy analysis. All enrolled patients had moderately to severely active UC defined as aMs of 5 to 9 with an ESS of 2 or 3 and demonstrated prior treatment failure including inadequate response, loss of response, or intolerance to prior conventional and/or biologic treatment. U-ACHIEVE Maintenance (UC-3) was a multicenter, double-blind, placebo-controlled clinical study with 451 patients who achieved clinical response per aMs (decrease ≥2 points and ≥30% from baseline and a decrease in RBS ≥1 from baseline or an absolute RBS ≤1) with 8-week RINVOQ 45 mg QD induction treatment. Patients were rerandomized 1:1:1 to receive either RINVOQ 15 mg QD, 30 mg QD, or placebo.1,2
UP NEXT
RINVOQ is an oral, once daily, selective and reversible JAK inhibitor now approved for the treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.1
RINVOQ can be taken at any time of the day, with or without food.1
A Phase 3 clinical trial program involving 3 studies: 2 replicate induction studies and 1 maintenance study evaluated RINVOQ 45 mg QD vs placebo for induction and RINVOQ 15 mg QD and 30 mg QD vs placebo for maintenance treatment.1,2
[Please insert local summary of safety]
REFERENCES
- RINVOQ [Summary of Product Characteristics]. AbbVie Deutschland GmbH & Co. KG; December 2023.
- Danese S, Vermeire S, Zhou W, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399(10341):2113-2128. doi:10.1016/S0140-6736(22)00581-5
- Panaccione R, Blumenstein I, Irving P, et al. Benefit-risk assessment of upadacitinib treatment in patients with moderately to severely active ulcerative colitis. Poster presented at: United European Gastroenterology Week; October 8-11, 2022; Vienna, Austria. P243.