RINVOQ® (upadacitinib) is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis (UC) who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.1
RINVOQ achieved the primary endpoints of clinical remission per adapted Mayo score at Induction Week 8 and Maintenance Week 521,2
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
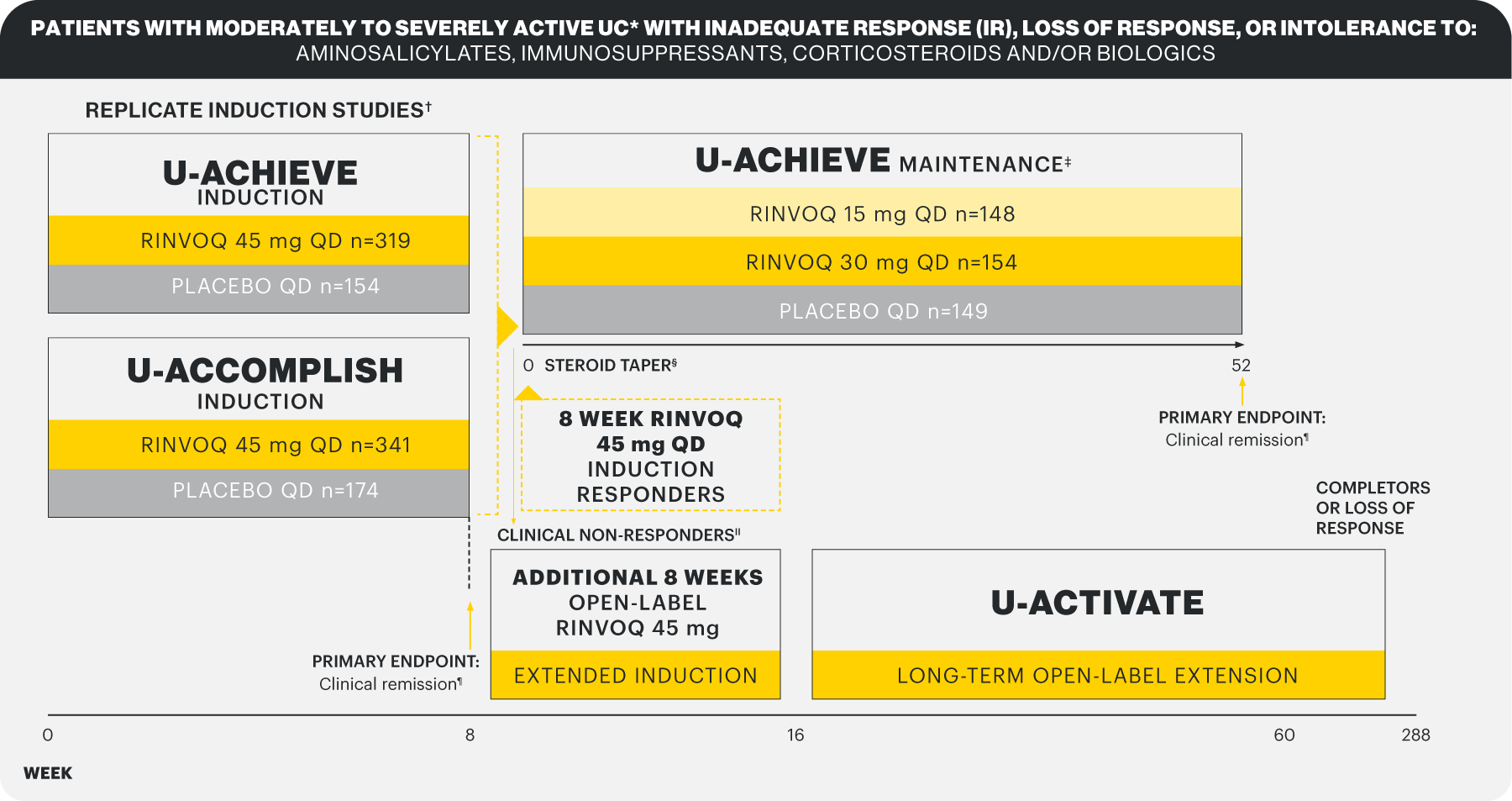
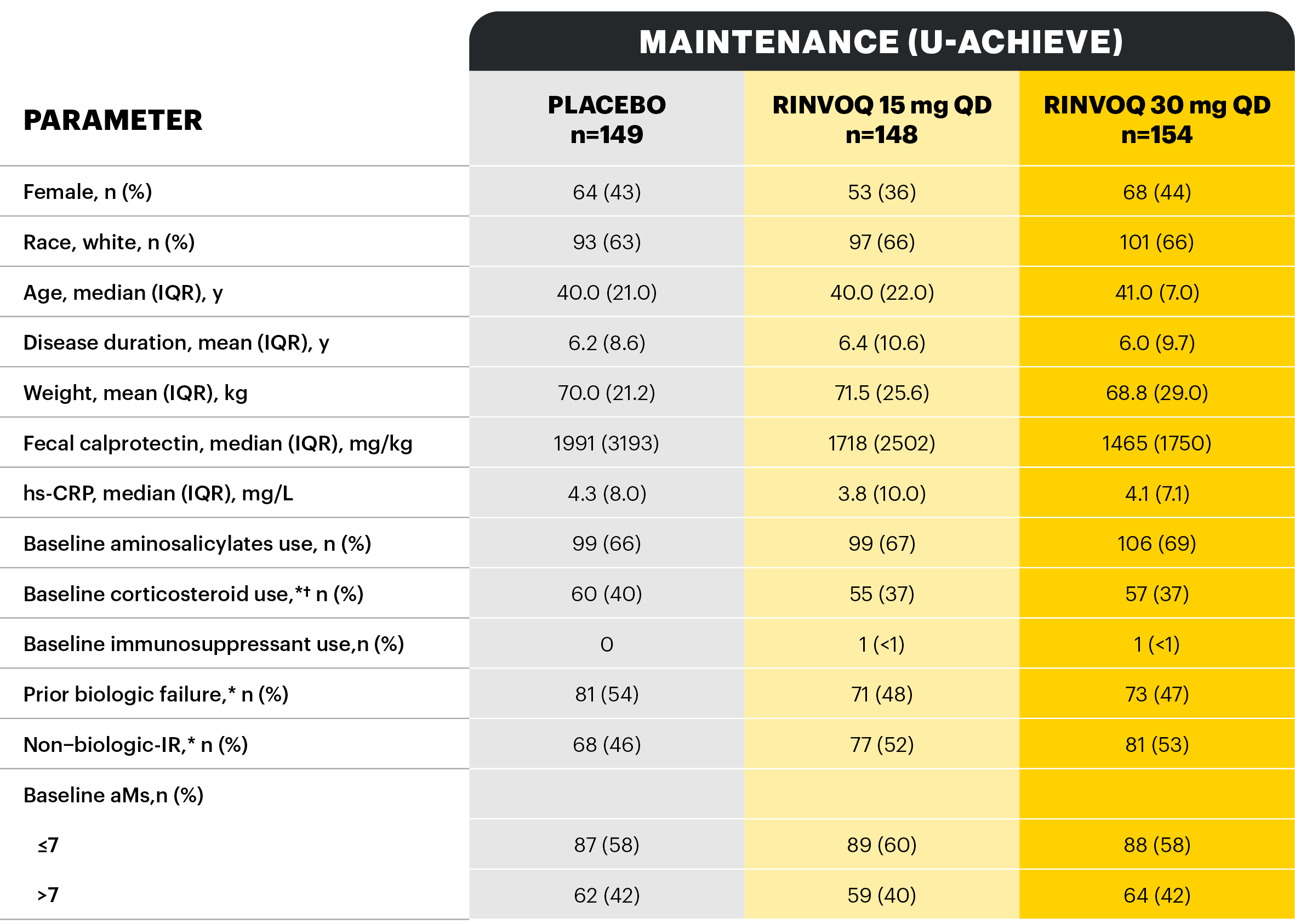
A Phase 3 trial program involving 3 studies: 2 replicate induction studies (U-ACHIEVE Induction and U-ACCOMPLISH) and 1 maintenance study (U-ACHIEVE Maintenance). A total of 988 patients with moderately to severely active UC evaluating RINVOQ 45 mg QD vs placebo for induction and RINVOQ 15 mg QD and 30 mg QD vs placebo for maintenance treatment (N=451).1*
*Patients who achieved clinical response per adapted Mayo score with 8-week RINVOQ 45 mg QD induction treatment entered maintenance.
*Defined as adapted Mayo Score 5–9 with centrally assessed endoscopic subscore of 2–3. †Randomization stratified by history of biologic failure (IR, loss of response or intolerance [yes vs no]), baseline corticosteroid use (yes vs no) and baseline adapted Mayo Score (≤7 vs >7). ‡Randomization stratified by previous biologic failure, induction clinical remission status, and corticosteroid use at the beginning of U-ACHIEVE Maintenance. Primary efficacy analysis = first 450 [planned] clinical responders to 8-week RINVOQ 45 mg induction. §For patients taking corticosteroid therapy at baseline of induction studies (~40%). At Week 0 of the maintenance study (Week 16 of the open-label extension), corticosteroid therapy was tapered according to a predefined schedule. IIPatients who did not achieve clinical response at Week 8 in U-ACHIEVE Induction and U-ACCOMPLISH continued in an additional 8-week, open-label, induction extension period with RINVOQ 45 mg QD. Patients who received 8 weeks total RINVOQ 45 mg QD with response at Week 16 were rerandomized into the maintenance study. Patients who received RINVOQ 45 mg QD for 16 weeks who had a response at Week 16 were randomized to RINVOQ 15 or 30 mg QD maintenance but were not included in the primary analysis. Patients who did not achieve clinical response by Week 16 of induction were discontinued. ¶Adapted Mayo score ≤2 with SFS ≤1 and not greater than baseline, RBS=0, PLUS ESS 0 or 1 without friability.
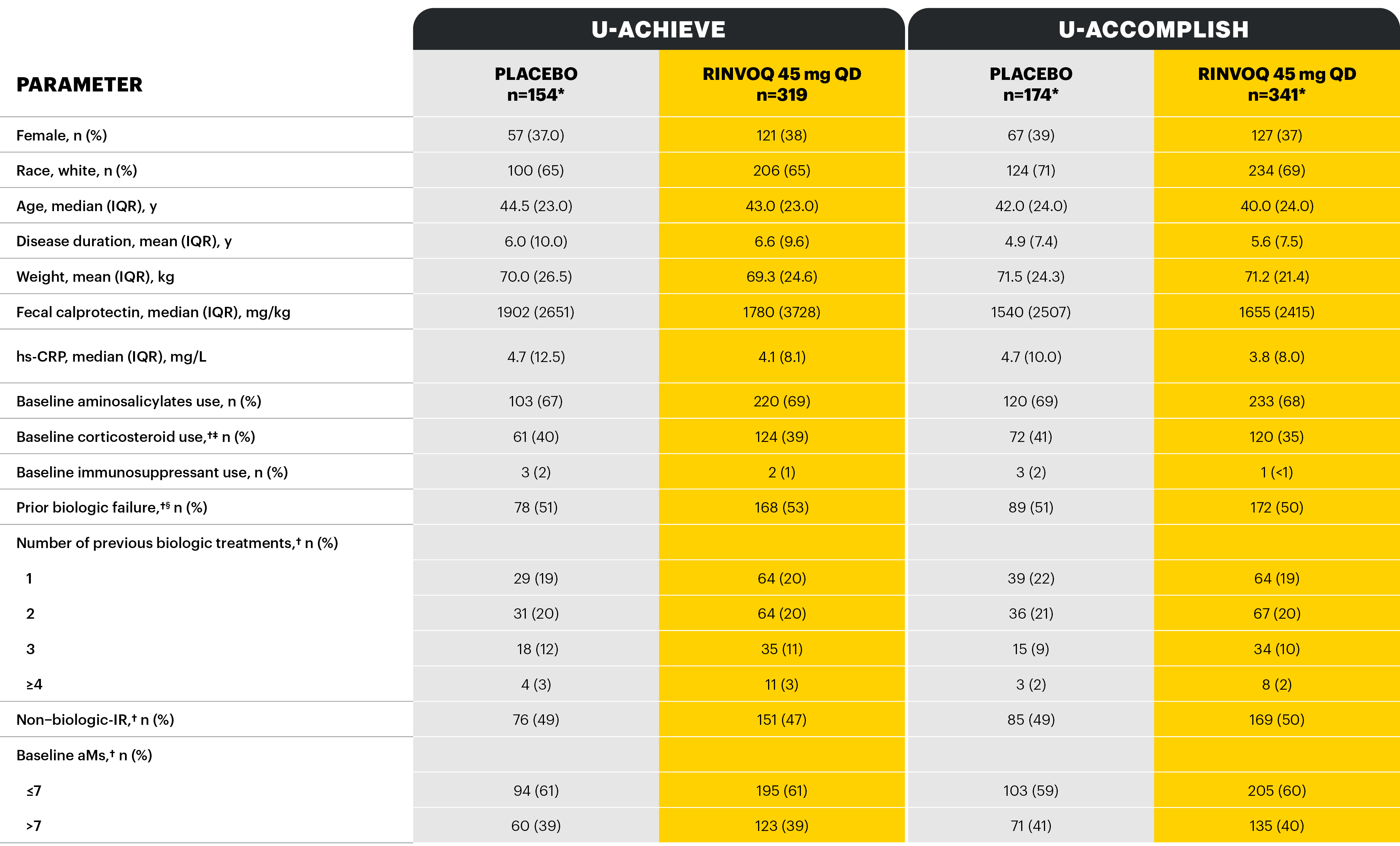
*One placebo patient in U-ACHIEVE Induction, 3 placebo patients from U-ACCOMPLISH, and 3 RINVOQ 45 mg patients from U-ACCOMPLISH were excluded from efficacy analyses because of site non-compliance; these patients were included in the safety analysis. †Stratification factors for randomization. ‡The highest allowed prednisolone dose at baseline was 30 mg. §Inadequate responder status, baseline steroid use, and baseline adapted Mayo score.
16 to 75 yearsa with a diagnosis of active UC for ≥90 days prior to baseline,b with an aMs of 5 to 9 points and ESS of 2 to 3
Inadequate response to, loss of response to, or intolerance to at least one of the following treatments including oral aminosalicylates, corticosteroids, immunosuppressants and/or biologic therapiesc
History of colectomy (total or subtotal, ileoanal pouch, Kock pouch, or ileostomy or were planning bowel surgery
aBody weight ≥40 kg at BL for subjects 16 and 17 years. bConfirmed by colonoscopy during the screening period, with exclusion of current infection, colonic dysplasia and/or malignancy. cAn inadequate response, loss of response, or intolerance to oral aminosalicylates did not count towards eligibility in Austria, Czechia, Finland, France, Ireland, Italy, Latvia, Lithuania, Norway, Poland, Portugal, Spain, Sweden, and the United Kingdom. dAt least one 6-week induction regimen of infliximab or vedolizumab, at least one 4-week induction regimen of adalimumab, at least one 2-week induction regimen of golimumab and at least one induction regimen of ustekinumab. eDiscontinuation despite clinical benefit does not qualify. fIncluding, but not limited to infusion-related reaction, demyelination, congestive heart failure and infection. gSystemic use of known strong cytochrome P450 CYP3A inhibitors or strong CYP3A inducers during the screening period and through to the end of the study. hOral or parenteral. iWithin 4 weeks or five half-lives of the drug (whichever is longer) or is currently enrolled in another clinical study.
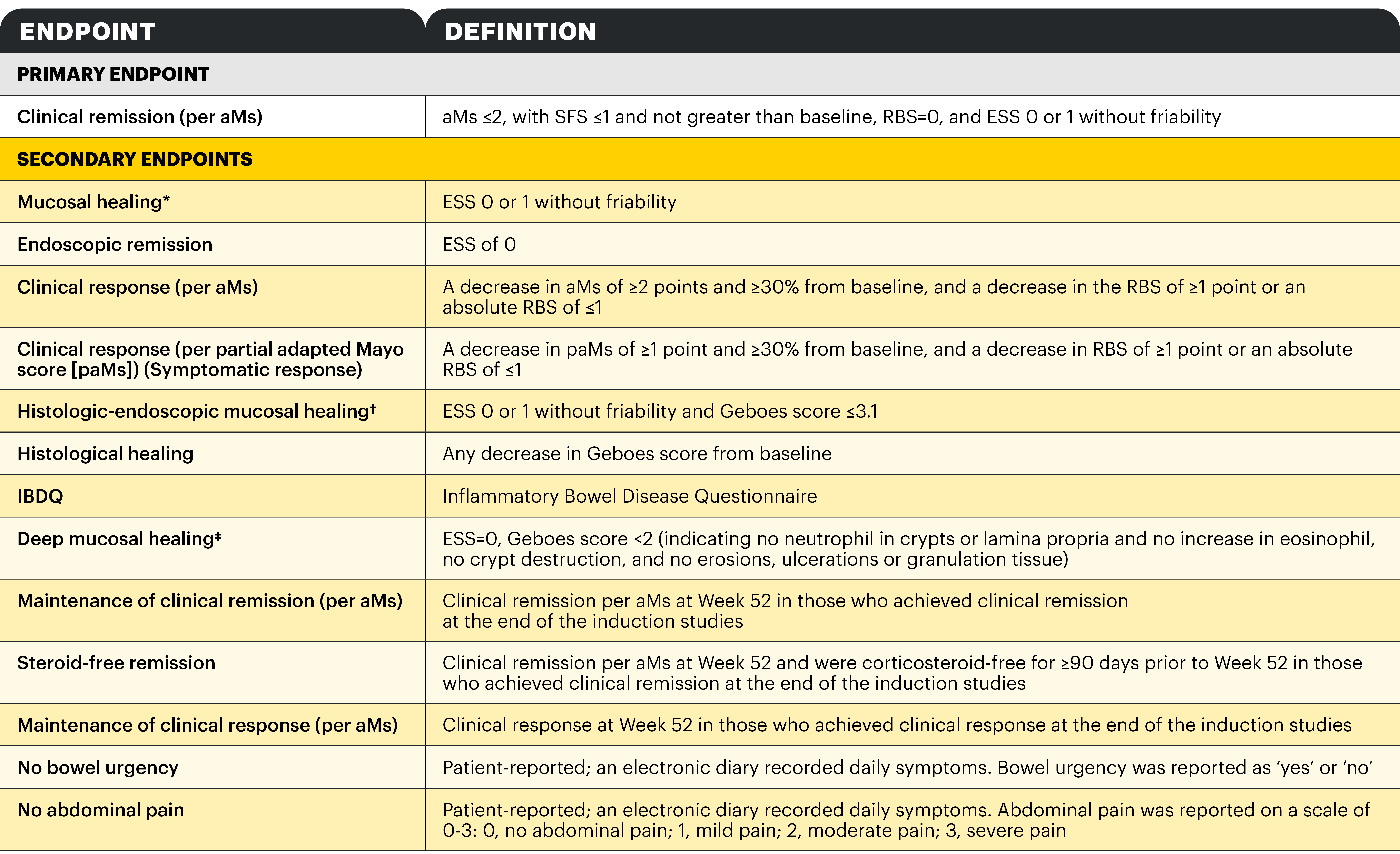
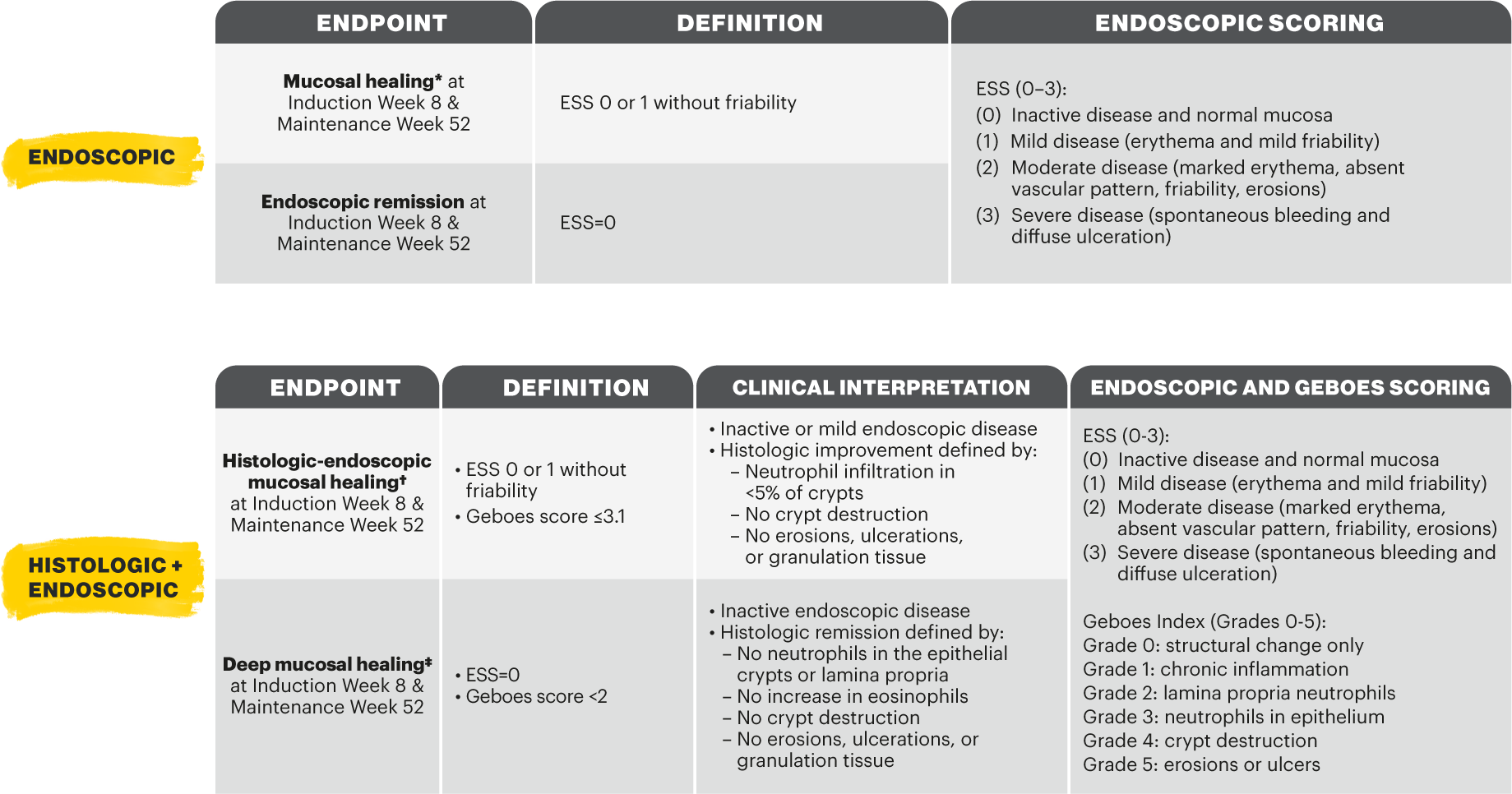
*Definition as per RINVOQ summary of product characteristics, the equivalent endpoint was referred to as 'endoscopic improvement' in the clinical study design.1,2 †Definition as per RINVOQ summary of product characteristics, the equivalent endpoint was referred to as 'histologic-endoscopic mucosal improvement' in the clinical study design.1,2 ‡Definition as per RINVOQ summary of product characteristics, the equivalent endpoint was referred to as 'mucosal healing' in the clinical study design.1,2
*Definition as per RINVOQ SmPC, the equivalent endpoint was referred to as 'endoscopic improvement' in the clinical study design.1,2 †Definition as per RINVOQ SmPC, the equivalent endpoint was referred to as 'histologic-endoscopic mucosal improvement' in the clinical study design.1,2 ‡Definition as per RINVOQ SmPC, the equivalent endpoint was referred to as 'mucosal healing' in the clinical study design.1,2
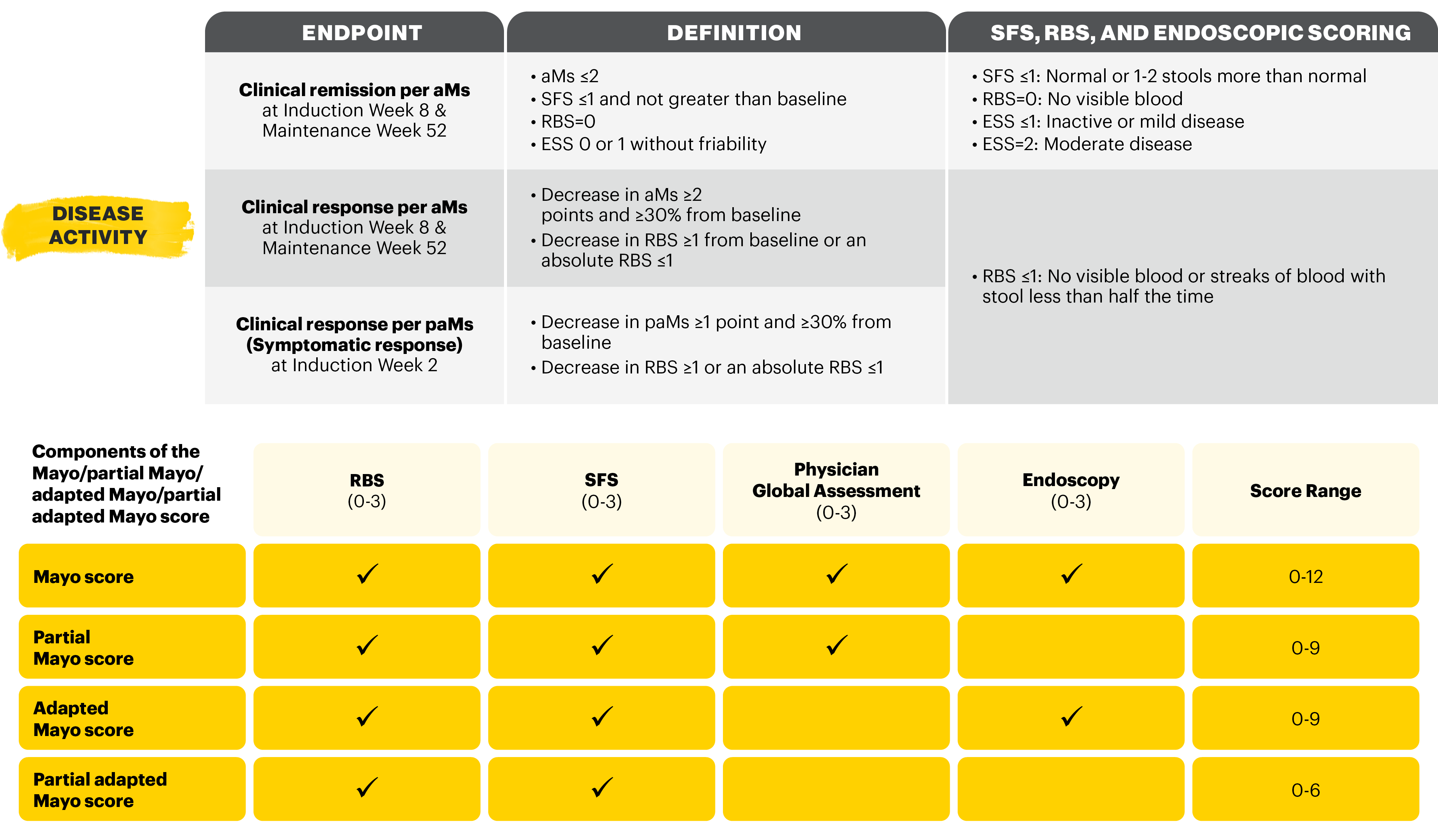
aMs: adapted Mayo score; BL: baseline; CRP: c-reactive protein; ESS: endoscopic subscore; IQR: interquartile range; IR: inadequate response; JAK: Janus kinase; paMs: partial adapted Mayo score; PGA: Physician Global Assessment; QD: once-daily; RBS: rectal bleeding score; SD: standard deviation; SFS: stool frequency score; UC: ulcerative colitis.
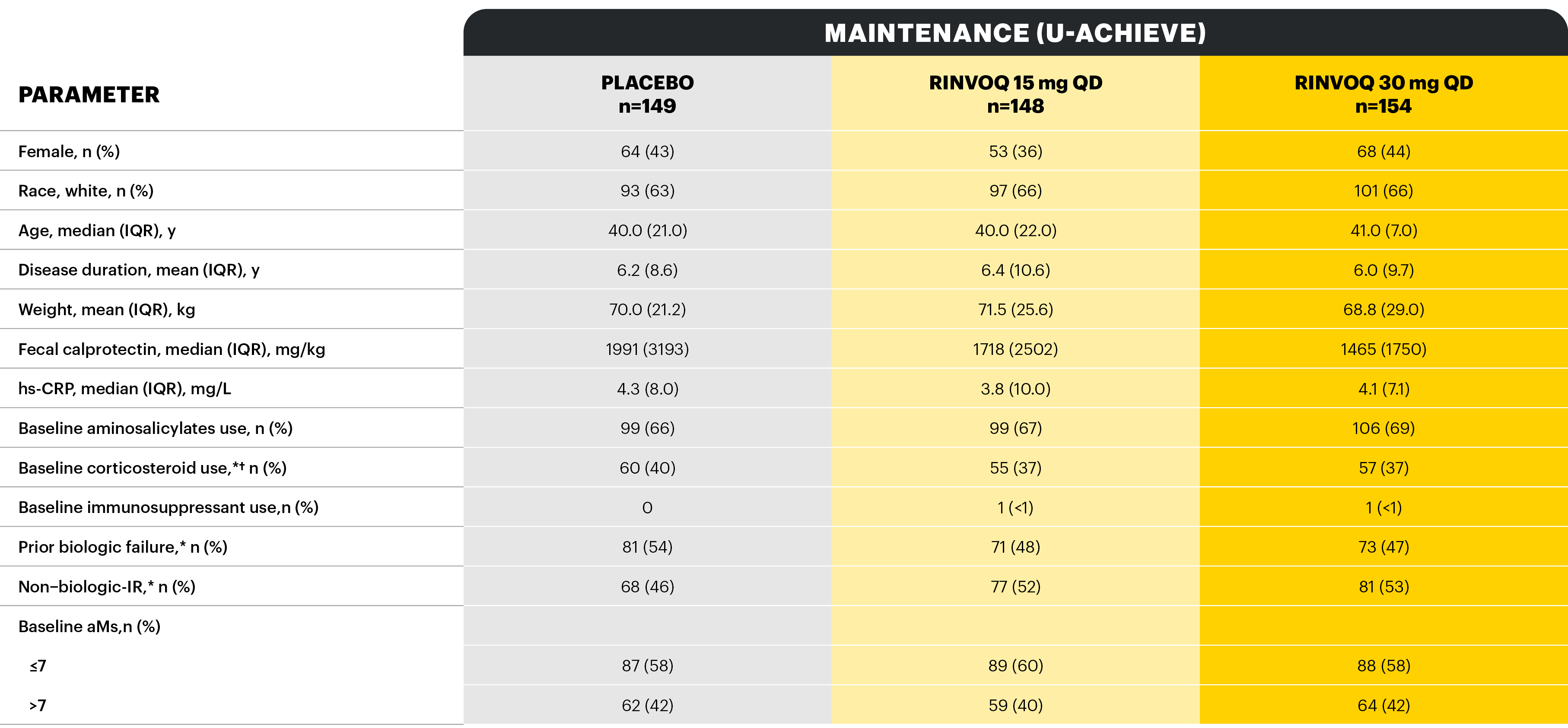
Study designs: U-ACHIEVE Induction (UC-1) and U-ACCOMPLISH (UC-2) were replicate induction studies, both of which were multicenter, double-blind, placebo-controlled clinical studies. In UC-1 and UC-2, 988 patients (473 and 515 patients, respectively) were randomized to RINVOQ 45 mg QD or placebo for 8 weeks with a 2:1 treatment allocation ratio and included in the efficacy analysis. All enrolled patients had moderately to severely active UC defined as aMs of 5 to 9 with an ESS of 2 or 3 and demonstrated prior treatment failure including inadequate response, loss of response, or intolerance to prior conventional and/or biologic treatment. U-ACHIEVE Maintenance (UC-3) was a multicenter, double-blind, placebo-controlled clinical study with 451 patients who achieved clinical response per aMs (decrease ≥2 points and ≥30% from baseline and a decrease in RBS ≥1 from baseline or an absolute RBS ≤1) with 8-week RINVOQ 45 mg QD induction treatment. Patients were rerandomized 1:1:1 to receive either RINVOQ 15 mg QD, 30 mg QD, or placebo.1,2
Clinical remission per aMs (primary endpoint): SFS ≤1 and not greater than baseline, RBS=0, ESS 0 or 1 without friability.
UP NEXT
RINVOQ is an oral, once daily, selective and reversible JAK inhibitor now approved for the treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.1
RINVOQ can be taken at any time of the day, with or without food. Tablets should be swallowed whole and should not be split, crushed, or chewed in order to ensure the entire dose is delivered correctly.1
A Phase 3 clinical trial program involving 3 studies: 2 replicate induction studies and 1 maintenance study evaluated RINVOQ 45 mg QD vs placebo for induction and RINVOQ 15 mg QD and 30 mg QD vs placebo for maintenance treatment.1,2
[Please insert local summary of safety]
REFERENCES
- RINVOQ [Summary of Product Characteristics]. AbbVie Deutschland GmbH & Co. KG; December 2023.
- Danese S, Vermeire S, Zhou W, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399(10341):2113-2128. doi:10.1016/S0140-6736(22)00581-5