RINVOQ® (upadacitinib) is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis (UC) who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.1
RINVOQ achieved the primary endpoints of clinical remission per adapted Mayo score at Induction Week 8 and Maintenance Week 521,2
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
A Phase 3 trial program involving 3 studies: 2 replicate induction studies (U-ACHIEVE Induction and U-ACCOMPLISH) and 1 maintenance study (U-ACHIEVE Maintenance). 988 moderately to severely active UC patients evaluating RINVOQ 45 mg QD vs placebo for induction and RINVOQ 15 mg QD and 30 mg QD vs placebo for maintenance treatment (N=451).1*
*Patients who achieved clinical response per adapted Mayo score with 8-week RINVOQ 45 mg QD induction treatment entered maintenance.
Ulcerative colitis is an unpredictable, chronic condition which can pose a significant risk to patients through its varying disease course.3
Uncontrolled UC can lead to increased risk of flares and relapse.4,5 Current ECCO guidelines state that courses of corticosteroids should be restricted to a maximum of 3 months.6
In order to induce and maintain clinical response and remission rates in the treatment of moderately to severely active UC, it is beneficial to look beyond disease symptoms when making clinical decisions, as per current STRIDE-II guidelines.7
Proportion of patients with clinical remission per aMs at Week 8 (stool frequency subscore ≤1 and not greater than baseline plus rectal bleeding subscore=0 plus endoscopic subscore 0 or 1 without friability)1,2
The primary endpoint of both induction studies was achievement of remission per adapted Mayo score at Week 8.1,2
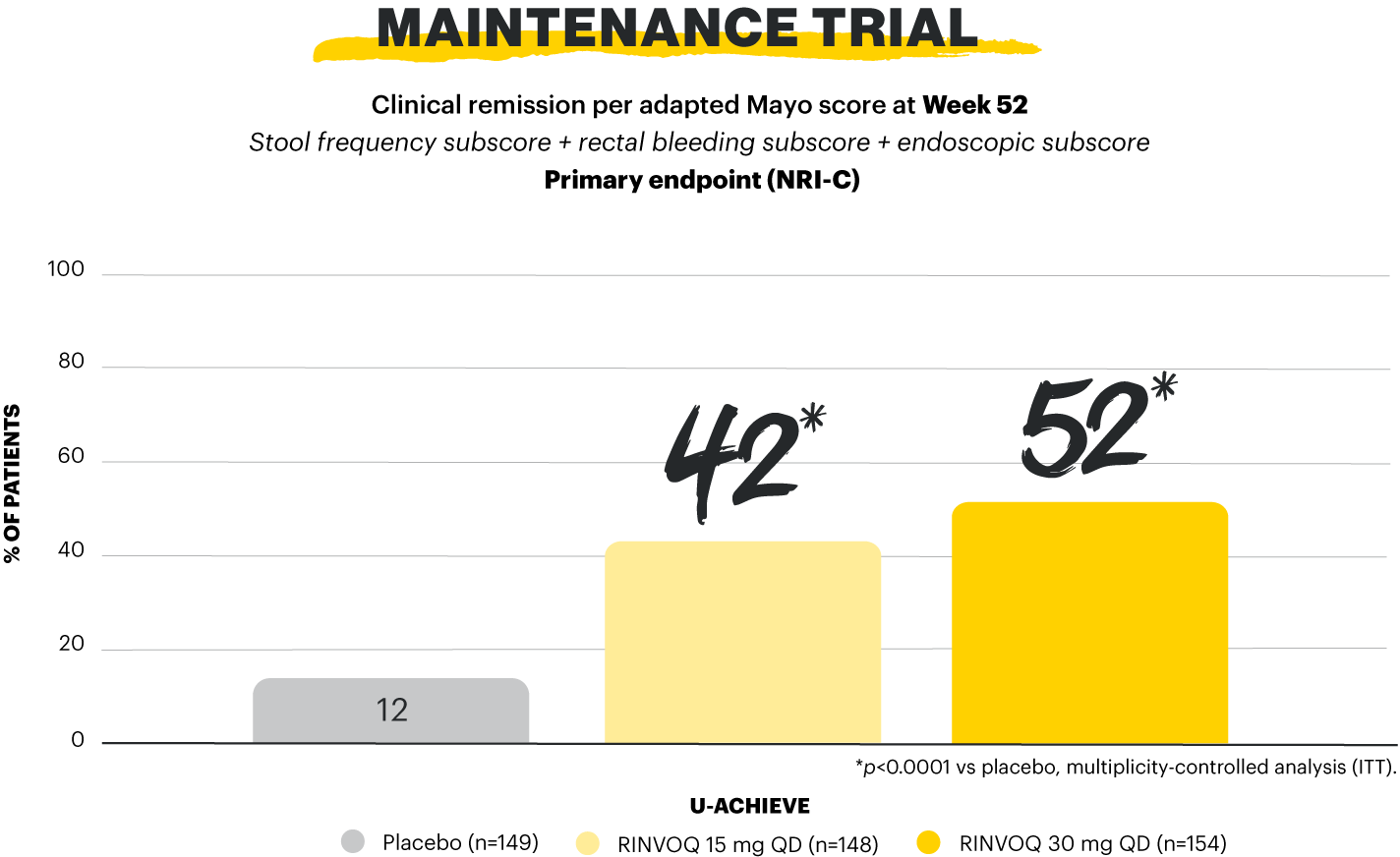
Clinical remission per aMs at Week 52 was the primary endpoint in the maintenance study.1,2
aMs: adapted Mayo score; ECCO: European Crohn's and Colitis Organisation; ESS: endoscopic subscore; ITT: intention to treat; NRI-C: non-responder imputation incorporating multiple imputations to handle missing data due to coronavirus disease 2019 (COVID-19); QD: once daily; RBS: rectal bleeding score; SFS: stool frequency score; STRIDE: Selecting Therapeutic Targets in Inflammatory Bowel Disease; UC: ulcerative colitis.
Study designs: U-ACHIEVE Induction (UC-1) and U-ACCOMPLISH (UC-2) were replicate induction studies, both of which were multicenter, double-blind, placebo-controlled clinical studies. In UC-1 and UC-2, 988 patients (473 and 515 patients, respectively) were randomized to RINVOQ 45 mg QD or placebo for 8 weeks with a 2:1 treatment allocation ratio and included in the efficacy analysis. All enrolled patients had moderately to severely active UC defined as aMs of 5 to 9 with an ESS of 2 or 3 and demonstrated prior treatment failure including inadequate response, loss of response, or intolerance to prior conventional and/or biologic treatment. U-ACHIEVE Maintenance (UC-3) was a multicenter, double-blind, placebo-controlled clinical study with 451 patients who achieved clinical response per aMs (decrease ≥2 points and ≥30% from baseline and a decrease in RBS ≥1 from baseline or an absolute RBS ≤1) with 8-week RINVOQ 45 mg QD induction treatment. Patients were rerandomized 1:1:1 to receive either RINVOQ 15 mg QD, 30 mg QD, or placebo.1,2
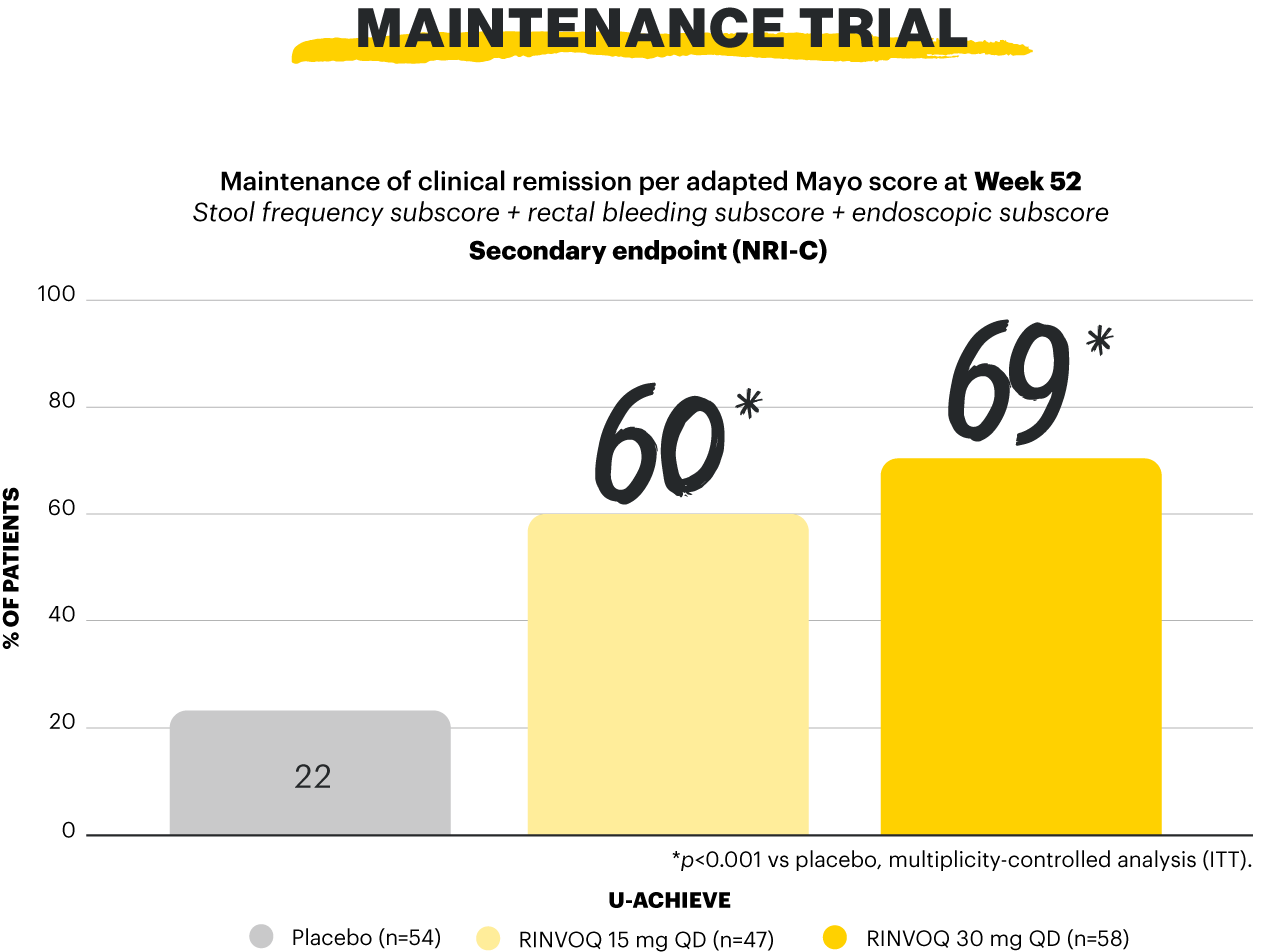
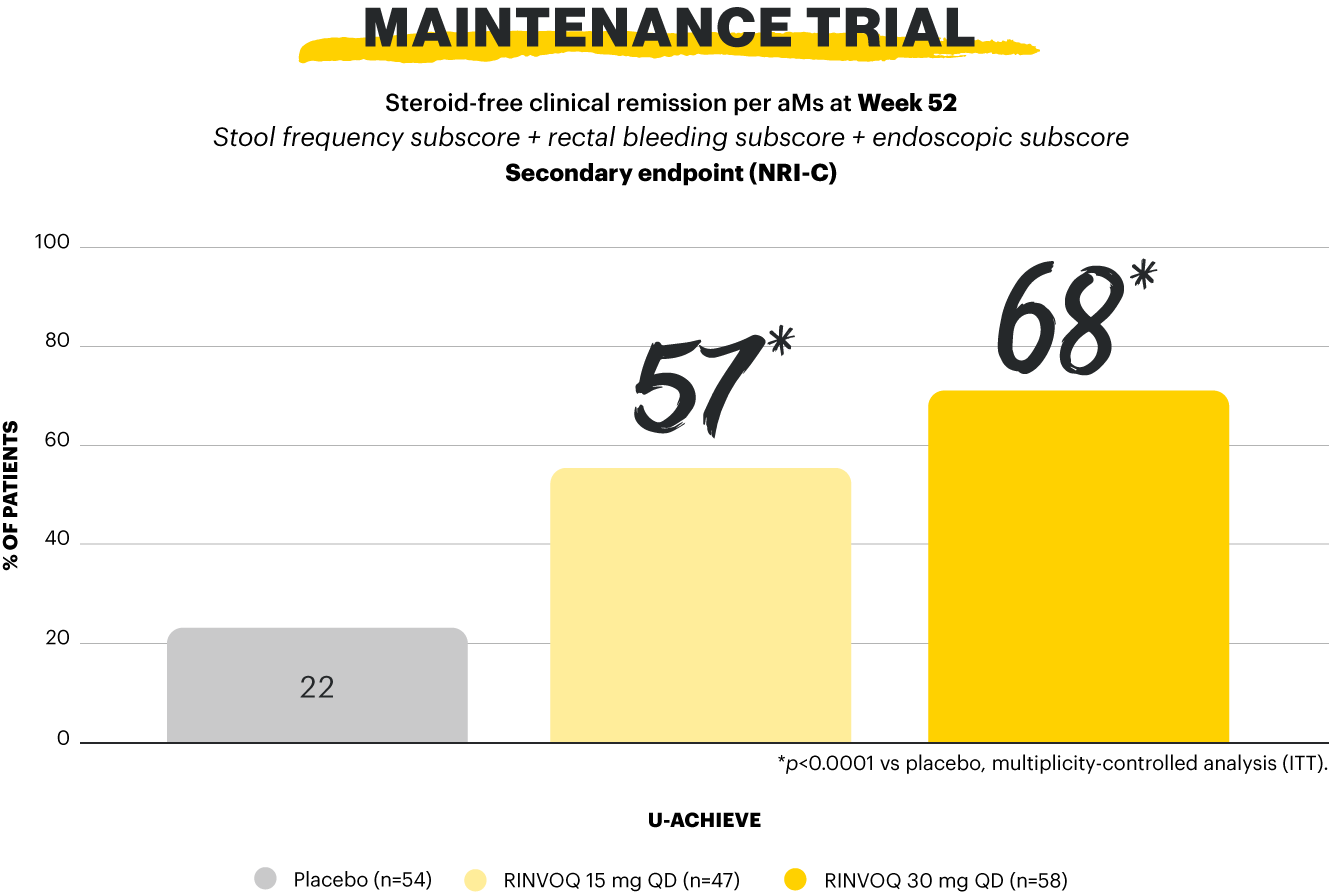
Clinical remission per aMs (primary endpoint): SFS ≤1 and not greater than baseline, RBS=0, ESS 0 or 1 without friability. Maintenance of clinical remission per aMs (secondary, multiplicity-controlled endpoint): clinical remission per aMs at Week 52 among patients who achieved clinical remission per aMs at Week 8 of RINVOQ 45 mg induction treatment (n=159). Steroid-free clinical remission (secondary, multiplicity-controlled endpoint): clinical remission per aMs at Week 52 and corticosteroid-free for ≥90 days immediately preceding Week 52 among patients who achieved clinical remission at the end of the induction treatment.1,2
[Please insert local summary of safety]
REFERENCES
- RINVOQ [Summary of Product Characteristics]. AbbVie Deutschland GmbH & Co. KG; Deember 2023.
- Danese S, Vermeire S, Zhou W, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399(10341):2113-2128. doi:10.1016/S0140-6736(22)00581-5
- Lichtenstein GR, Rutgeerts P. Importance of mucosal healing in ulcerative colitis. Inflamm Bowel Dis. 2010;16(2):338-346. doi:10.1002/ibd.20997
- Atreya R, Neurath MF. Current and future targets for mucosal healing in inflammatory bowel disease. Visc Med. 2017;33(1):82-88. doi:10.1159/000458006
- Matsuoka K, Igarashi A, Sato N, et al. Trends in corticosteroid prescriptions for ulcerative colitis and factors associated with long-term corticosteroid use: analysis using Japanese claims data from 2006 to 2016. J Crohns Colitis. 2021;15(3):358-366. doi:10.1093/ecco-jcc/jjaa172
- Raine T, Bonovas S, Burisch J, et al. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohns Colitis. 2022;16(1):2-17. doi:10.1093/ecco-jcc/jjab178
- Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570-1583. doi:10.1053/j.gastro.2020.12.031