RINVOQ® (upadacitinib) is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis (UC) who have had an inadequate score, lost response or were intolerant to either conventional therapy or a biologic agent.1
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
AEs OF SPECIAL INTEREST PER 100 PYs IN PATIENTS WITH UC ENROLLED TO RECEIVE MAINTENANCE TREATMENT WITH RINVOQ FOR UP TO 52 WEEKS2
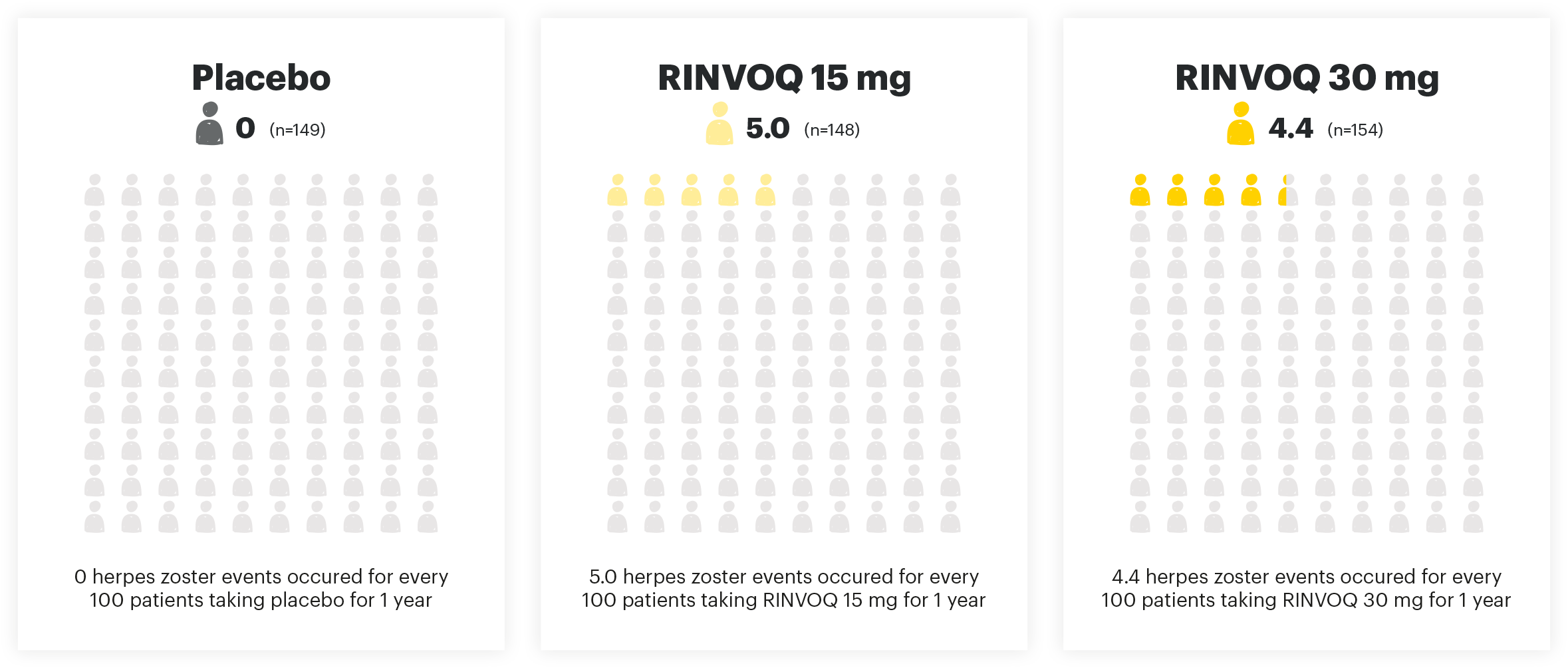
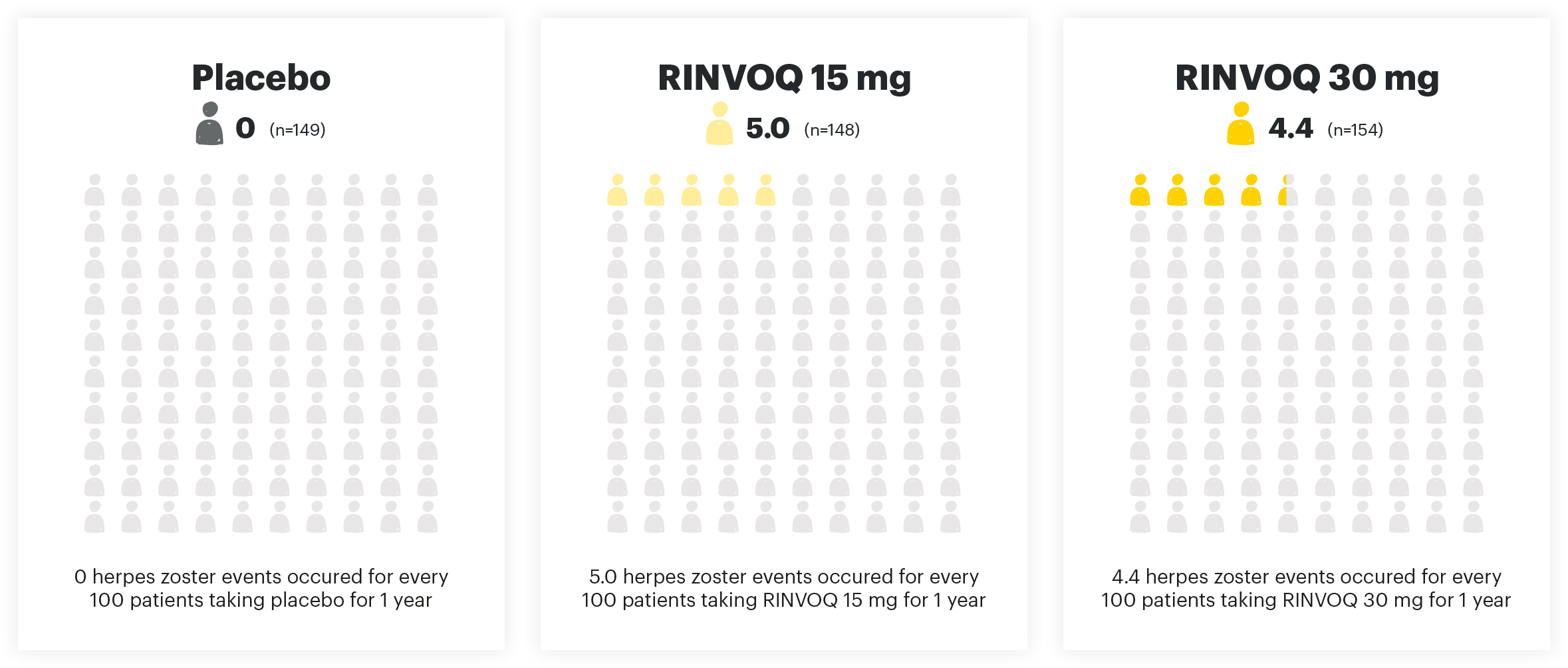
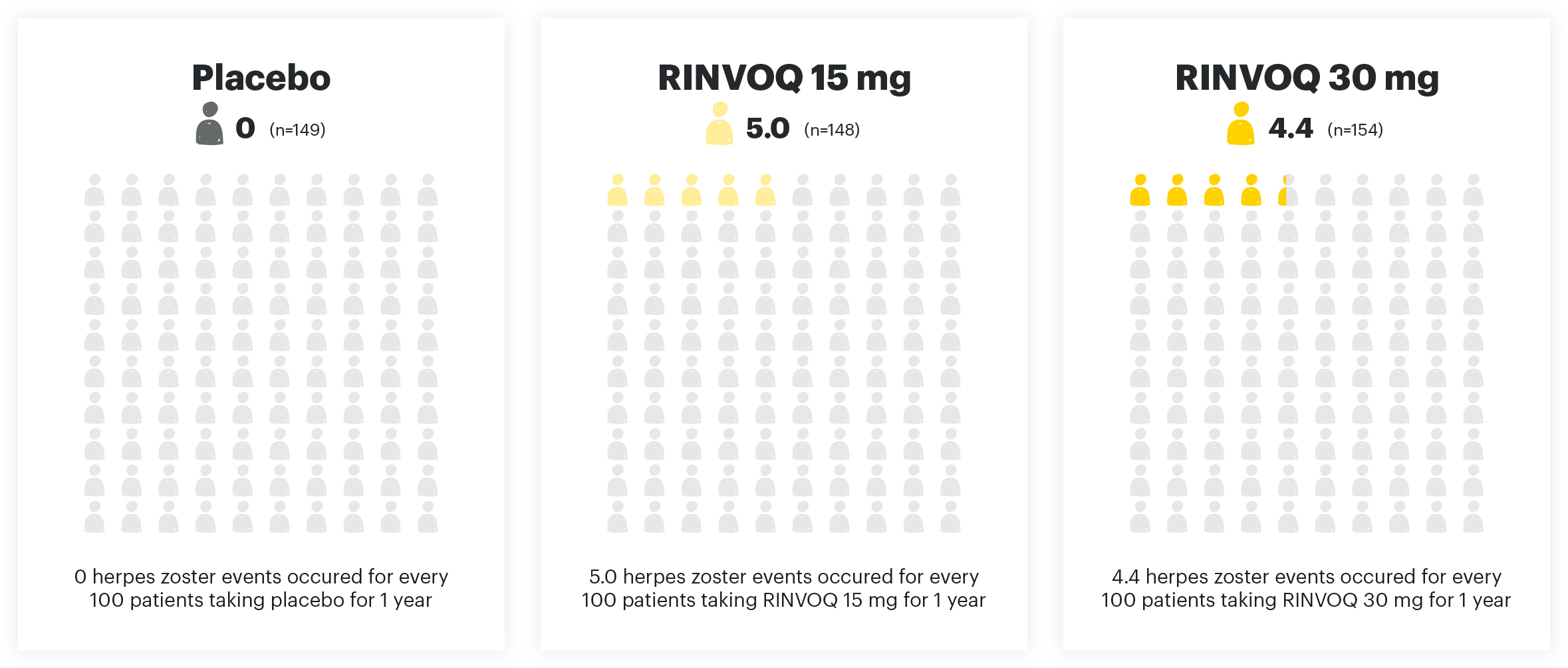
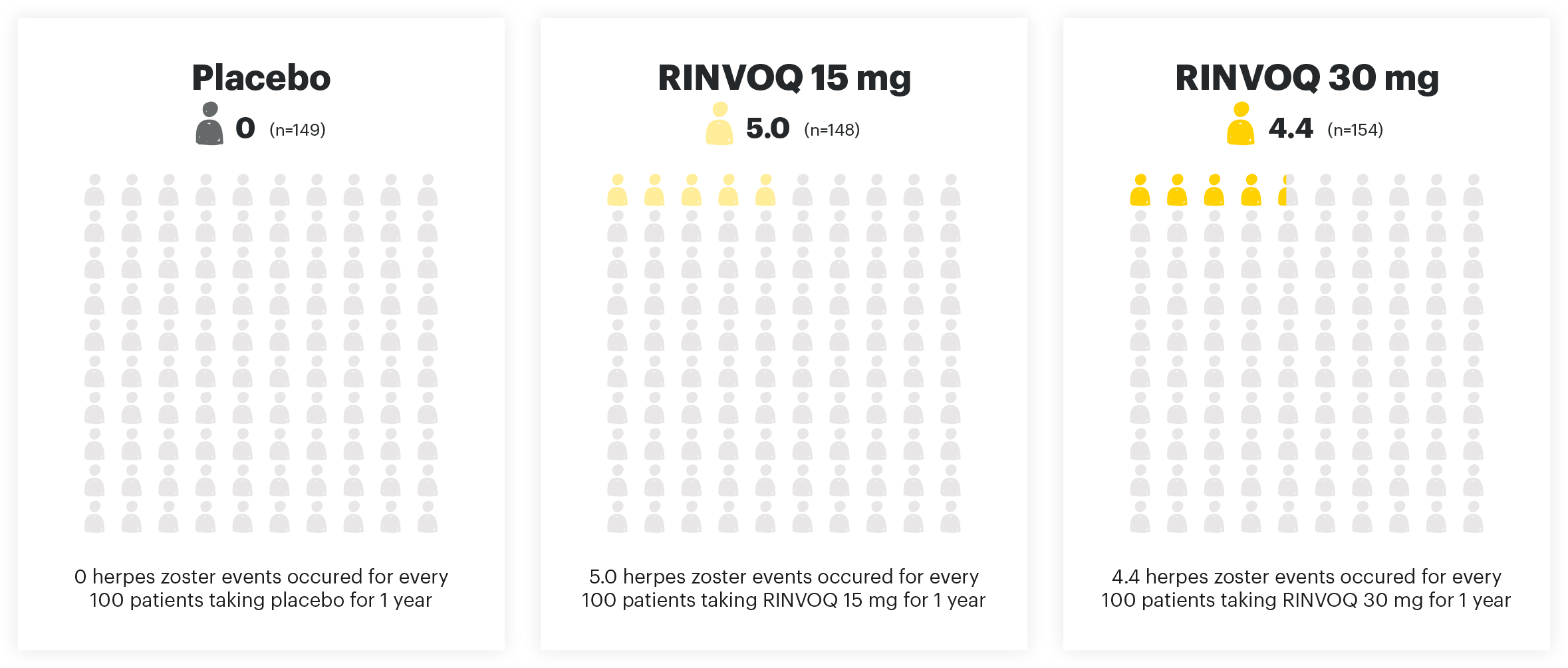
HERPES ZOSTER EVENTS PER 100 PYs
Adapted from Danese et al. 2022. The safety analysis population (n=451) in the maintenance study included all patients who received at least one dose of treatment as part of the primary analysis population.
RINVOQ Summary of Product Characteristics (SmPC)
Viral reactivation:
The risk of herpes zoster appears to be higher in Japanese patients treated with RINVOQ. If a patient develops herpes zoster, interruption of RINVOQ therapy should be considered until the episode resolves. Prior to initiating RINVOQ, it is recommended that patients be brought up to date with all immunizations, including prophylactic zoster vaccinations, in agreement with current immunization guidelines.1
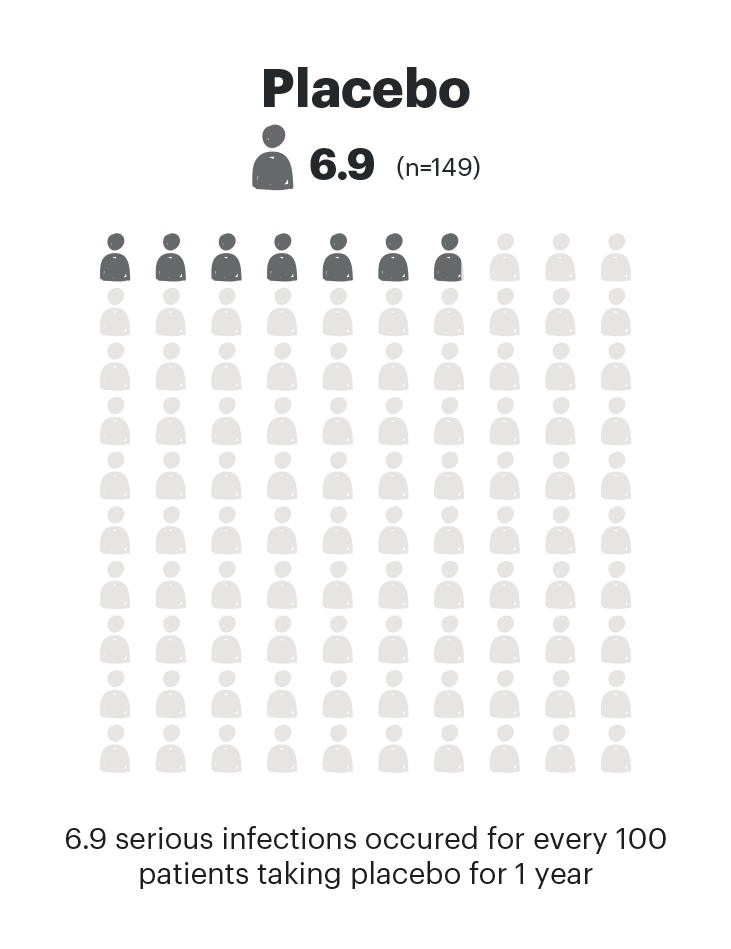
SERIOUS INFECTIONS EVENTS PER 100 PYs
Adapted from Danese et al. 2022. The safety analysis population (n=451) in the maintenance study included all patients who received at least one dose of treatment as part of the primary analysis population.
RINVOQ SmPC
Serious infections:
RINVOQ should not be initiated in patients with an active, serious infection, including localized infections. Treatment with RINVOQ therapy should be interrupted if a patient develops a serious or opportunistic infection. A higher rate of serious infections was observed with RINVOQ 30 mg compared to RINVOQ 15 mg. As there is a higher incidence of infections in the elderly ≥65 years of age and in the diabetic population in general, caution should be used when treating the elderly and patients with diabetes. In patients 65 years of age and older, RINVOQ should only be used if no suitable treatment alternatives are available.1
MALIGNANCY (EXLUDING NMSC) EVENTS PER 100 PYs
Adapted from Danese et al. 2022. The safety analysis population (n=451) in the maintenance study included all patients who received at least one dose of treatment as part of the primary analysis population.
RINVOQ SmPC
Malignancy:
Lymphoma and other malignancies have been reported in patients receiving Janus kinase (JAK) inhibitors, including RINVOQ. A higher rate of malignancies was observed with RINVOQ 30 mg compared to RINVOQ 15 mg. In patients 65 years of age and older, patients who are current or past long-time smokers, or patients with other malignancy risk factors (e.g., current malignancy or history of malignancy), RINVOQ should only be used if no suitable treatment alternatives are available.1
MACE (ADJUDICATED) EVENTS PER 100 PYs
Adapted from Danese et al. 2022. The safety analysis population (n=451) in the maintenance study included all patients who received at least one dose of treatment as part of the primary analysis population.
RINVOQ SmPC
MACE:
Events of MACE were observed in clinical studies of RINVOQ. In patients 65 years of age and older, patients who are current or past long-time smokers, and patients with history of atherosclerotic cardiovascular disease or other cardiovascular risk factors, RINVOQ should only be used if no suitable treatment alternatives are available.1
VTEs (ADJUDICATED) EVENTS PER 100 PYs
Adapted from Danese et al. 2022. The safety analysis population (n=451) in the maintenance study included all patients who received at least one dose of treatment as part of the primary analysis population.
RINVOQ SmPC
VTE:
Events of deep venous thrombosis and pulmonary embolism were observed in clinical trials for RINVOQ. In patients with cardiovascular or malignancy risk factors, RINVOQ should only be used if no suitable treatment alternatives are available. In patients with known VTE risk factors other than cardiovascular or malignancy risk factors, RINVOQ should be used with caution. VTE risk factors other than cardiovascular or malignancy risk factors include previous VTE patients undergoing major surgery, immobilization, use of combined hormonal contraceptives or hormone replacement therapy, and inherited coagulation disorder. Patients should be re-evaluated periodically during RINVOQ treatment to assess for changes in VTE risk. Promptly evaluate patients with signs and symptoms of VTE and discontinue RINVOQ in patients with suspected VTE, regardless of dose.1
AE: adverse event; JAK: Janus kinase; MACE: major adverse cardiovascular event; NMSC: non-melanoma skin cancer; PY: patient-year; UC: ulcerative colitis; VTE: venous thromboembolic event.
REFERENCES
- RINVOQ Summary of Product Characteristics.
- Danese S, Vermeire S, Zhou W, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399(10341):2113–2128.