DRAFT FOR INTERNAL USE ONLY
This DRAFT of the Global RINVOQ CD AbbVie Pro (v0.2) has been developed based on the DRAFT RINVOQ CD CLM, ALL-RNQG-220127 (v1.0).
RINVOQ CD was approved by the European Commission in April 2023 and the DRAFT CLM is being updated accordingly but is not final approved. When implemented locally, affiliates must align AbbVie Pro to the forthcoming final approved RINVOQ CD CLM, ALL-RNQG-220127 (version to be determined).
Note to Affiliates: Please evaluate use of ALL claims, graphs/tables, and corresponding references (e.g., data on file, abstracts, posters, manuscripts) according to local standards, codes, and regulations.
RINVOQ® (upadacitinib) is indicated for the treatment of adult patients with moderately to severely active Crohnʼs disease (CD) who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.1
RINVOQ achieved its co-primary endpoints of endoscopic response and clinical remission at Week 12 (Induction) and Week 52 (Maintenance)1
▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
A Phase 3 trial program involving 3 studies:1
2 induction studies (U-EXCEL and U-EXCEED) and 1 maintenance study (U-ENDURE). 1,021 moderately to severely active CD patients evaluating RINVOQ 45 mg QD vs placebo for induction, and RINVOQ 15 mg QD or 30 mg QD vs placebo for maintenance treatment (N=502).1*
*Patients who achieved clinical response (≥30% decrease in average daily stool frequency and/or in abdominal pain score, neither worse than baseline) to 12 weeks of RINVOQ 45 mg QD induction treatment entered maintenance.
ENDOSCOPIC OUTCOMES, INCLUDING MUCOSAL HEALING, ARE IMPORTANT IN THE TREATMENT OF CROHN'S DISEASE
Endoscopic healing is a STRIDE-II long-term target of CD treatment.2 In a Phase 3 clinical trial program evaluating the efficacy and safety of RINVOQ in CD, 5 different endpoints were used to measure endoscopic healing: endoscopic response, endoscopic remission, SES-CD 0–2, mucosal healing, and deep remission.1
Endoscopic response:
Decrease in SES-CD >50% from baseline of the induction study (or for patients with an SES-CD of 4 at baseline of the induction study, at least a 2-point reduction from baseline of the induction study)
Endoscopic remission:
SES-CD ≤4 and at least a 2-point reduction vs baseline and no subscore >1 in any individual variable
SES-CD 0–2:
SES-CD ulcerated surface subscore of 0–2 achieved in patients
Mucosal healing:
SES-CD ulcerated surface subscore of 0 in patients with SES-CD ulcerated surface subscore ≥1 at baseline
Deep remission:
Clinical remission (average daily SF ≤2.8 and APS ≤1.0 and neither greater than baseline) AND endoscopic remission (defined above)
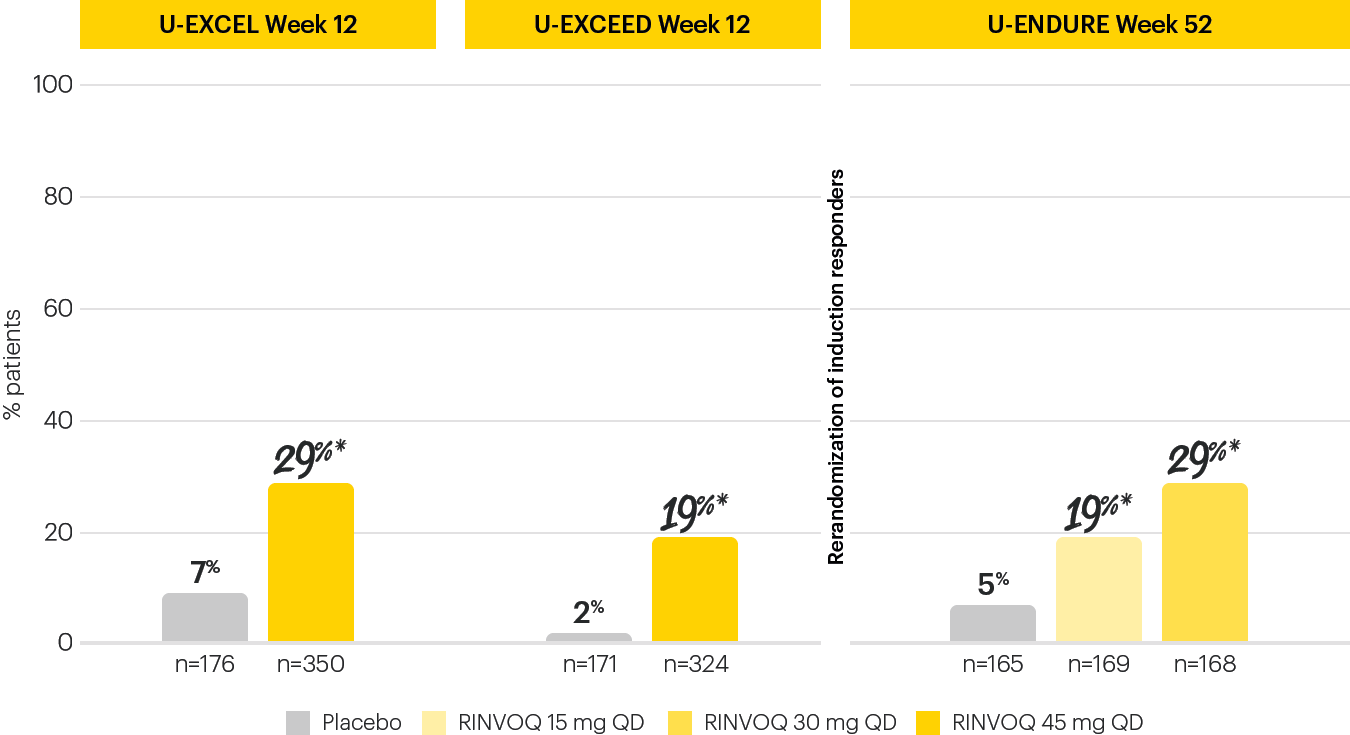
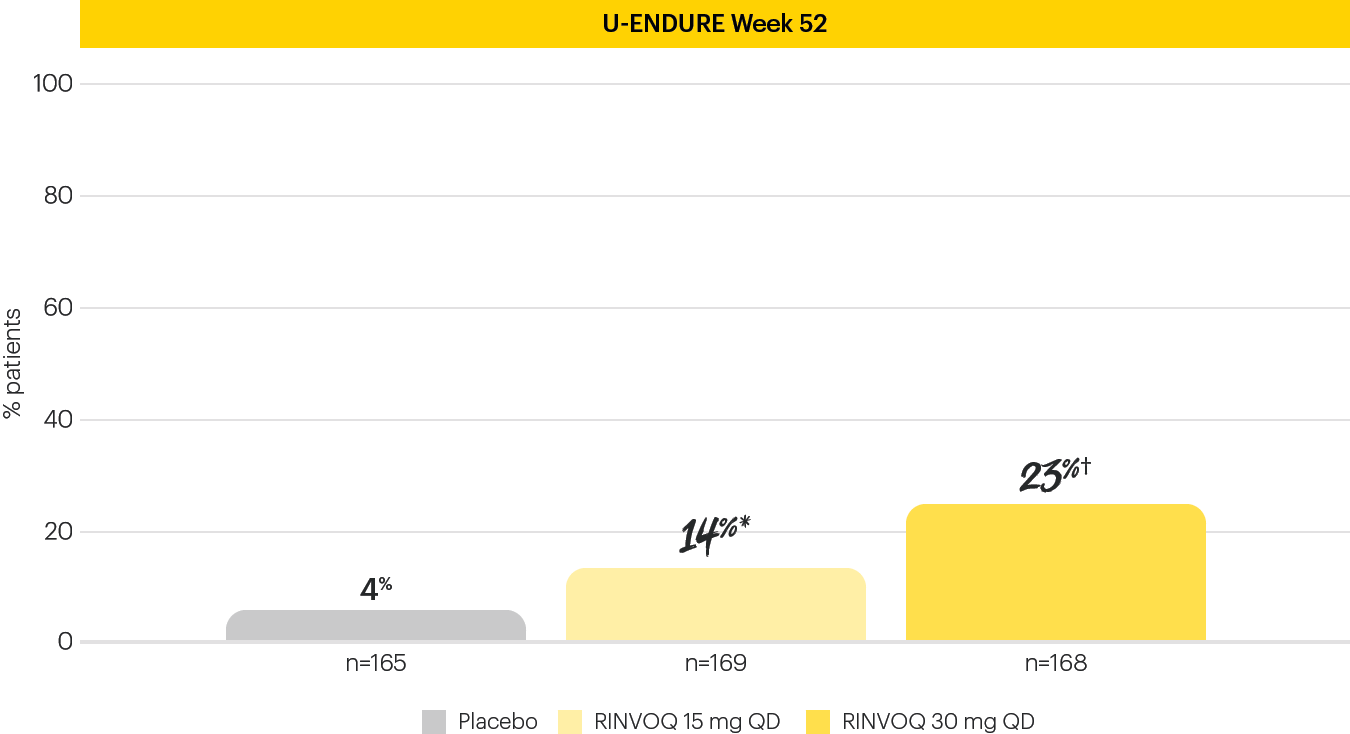
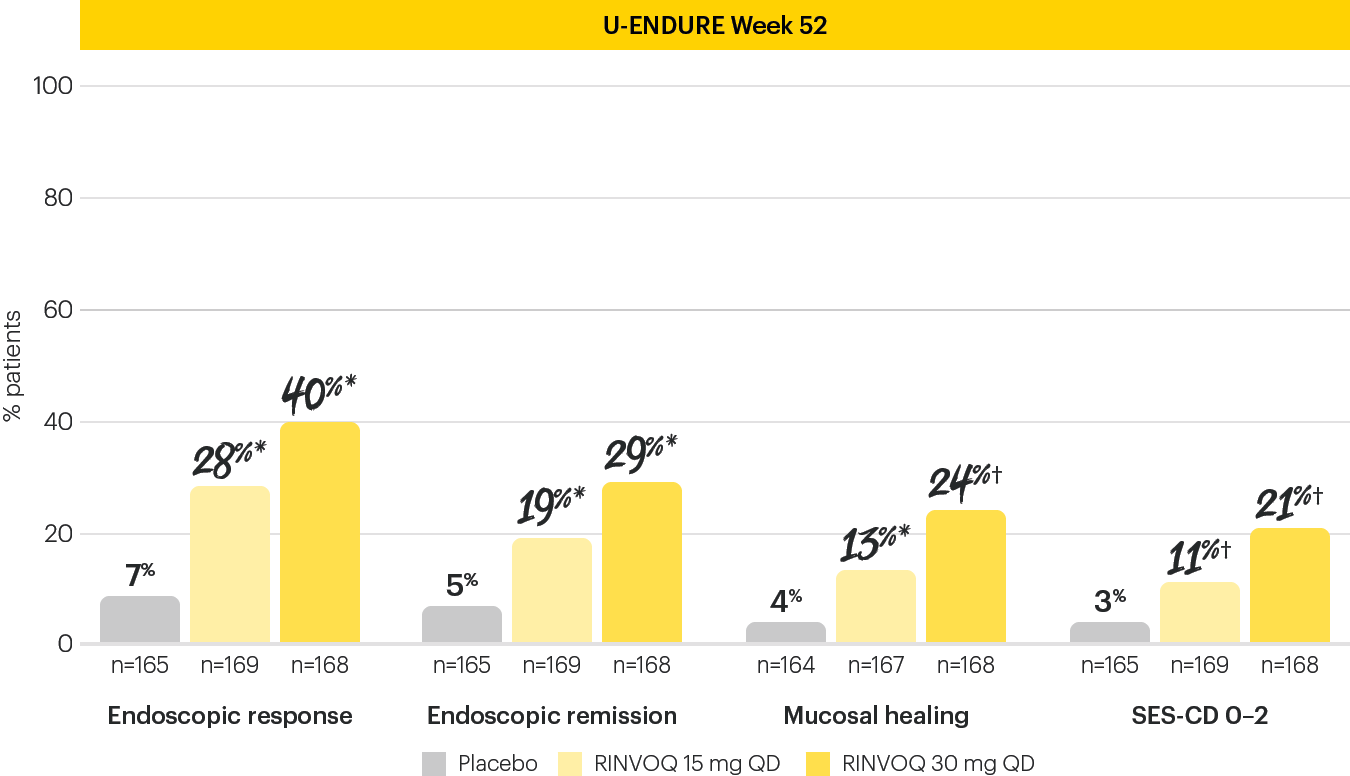
EARLY AND LONG-TERM ENDOSCOPIC REMISSION1
Endoscopic remission achieved at Induction Week 12 and Maintenance Week 521
The Week 12 analysis evaluated randomized patients who received at least one dose of study drug in the randomized induction.3 The efficacy analysis at Week 52 evaluated 502 patients who achieved clinical response (SF/APS) with the 12-week RINVOQ 45 mg QD induction treatment.1 Secondary, multiplicity-controlled endpoint.
*P<0.001; RINVOQ vs placebo.
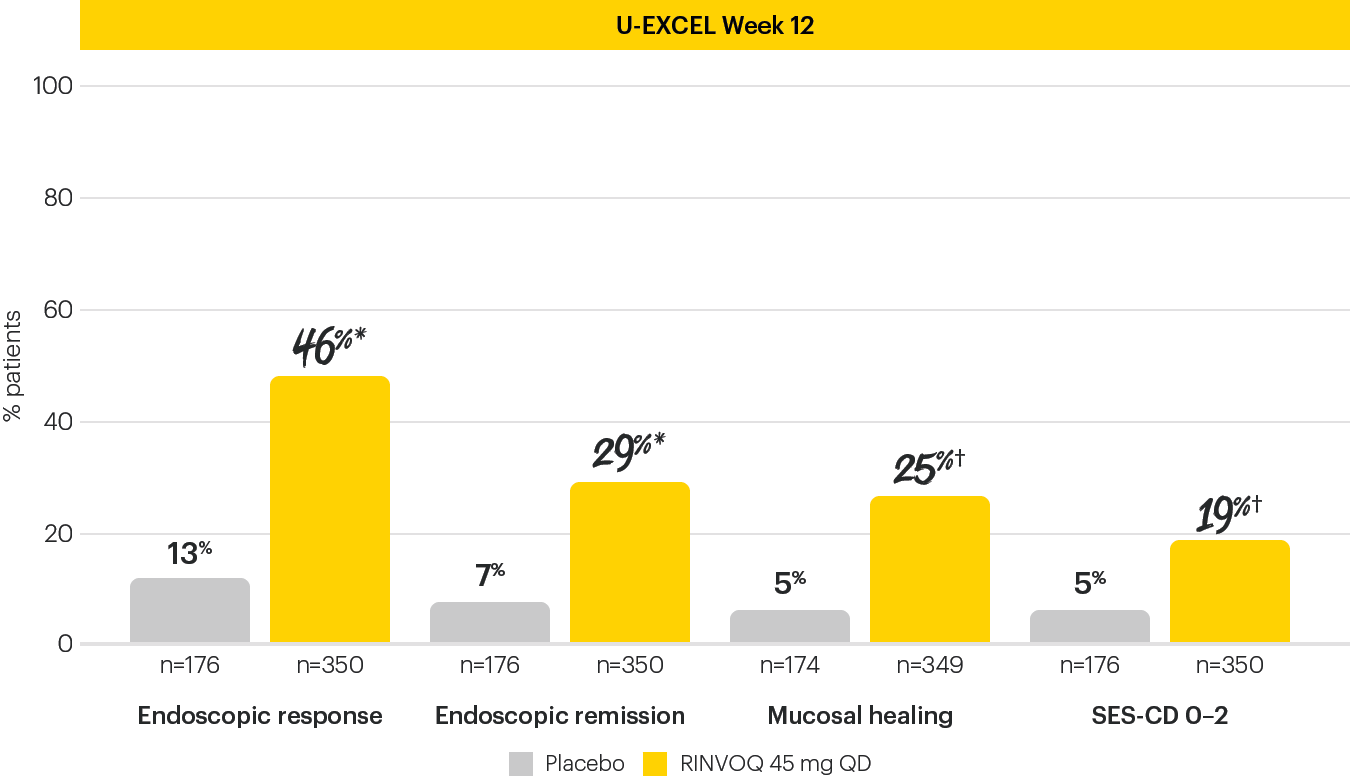
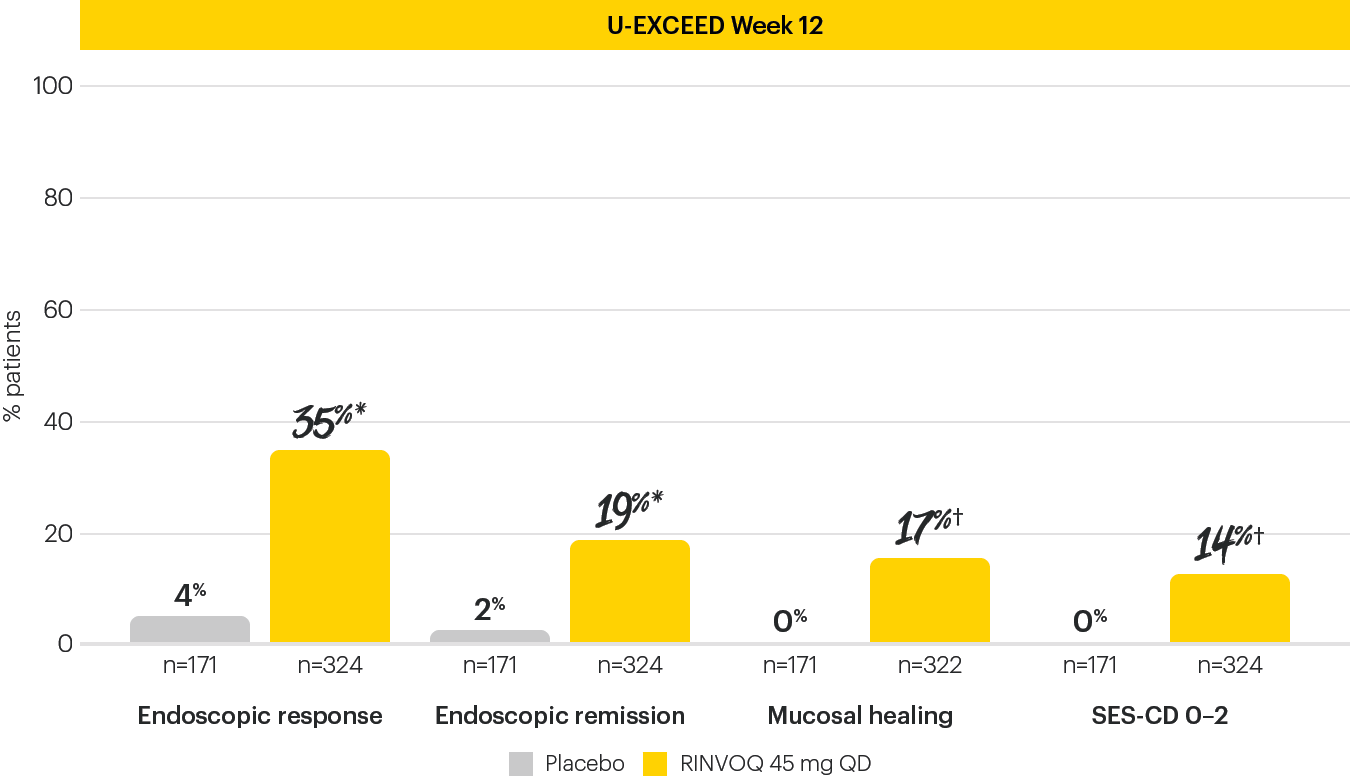
ENDOSCOPIC ASSESSMENTS AT INDUCTION WEEK 121,4
The Week 12 analysis evaluated randomized patients who received at least one dose of study drug in the randomized induction.3
Data limitations: Mucosal healing and SES-CD 0–2 analyses are not powered or tested to demonstrate a statistically significant difference in treatment effect; no statistical inferences can be made due to the exploratory nature of the analyses.
*P<0.001; †nominal P<0.001; RINVOQ vs placebo.
The Week 12 analysis evaluated randomized patients who received at least one dose of study drug in the randomized induction.3
Data limitations: Mucosal healing and SES-CD 0–2 analyses are not powered or tested to demonstrate a statistically significant difference in treatment effect; no statistical inferences can be made due to the exploratory nature of the analyses..
*P<0.001; †nominal P<0.001; RINVOQ vs placebo.
APS: abdominal pain score; CDAI: Crohn’s Disease Activity Index; JAK: Janus kinase; QD: once daily; SES-CD: simple endoscopic activity score for Crohn’s disease; SF: stool frequency; STRIDE: Selecting Therapeutic Targets in Inflammatory Bowel Disease.
Study designs: the U-EXCEL and U-EXCEED induction studies were both multicenter, double-blind, placebo-controlled clinical studies. In U-EXCEL (N=526 [287 bio-naive, 239 biologic failures]) and U-EXCEED (N=495 biologic failures only), patients were randomized to RINVOQ 45 mg QD or placebo for 12 weeks with a 2:1 treatment allocation ratio and included in the efficacy analysis. In both studies, induction nonresponders were allowed to enter an additional 12-week open-label extended treatment period. All enrolled patients had moderately to severely active CD defined as SF ≥4 and/or APS ≥2, plus an SES-CD ≥6 (≥4 for patients with isolated ileal disease) excluding the narrowing component. U-ENDURE maintenance was a multicenter, double-blind, placebo-controlled clinical study with 502 patients who achieved clinical response (≥30% decrease in average daily SF and/or in APS, neither worse than baseline) to 12 weeks of RINVOQ 45 mg QD induction treatment. These patients were rerandomized 1:1:1 to receive either RINVOQ 15 mg QD, 30 mg QD, or placebo.1
Co-primary endpoint: endoscopic response at Week 12. Secondary, multiplicity-controlled endpoints: endoscopic remission at Week 12. Additional endpoints not controlled for multiplicity: mucosal healing at Week 12 and SES-CD 0–2 at Week 12. Endoscopic response (co-primary endpoint): decrease in SES-CD >50% from baseline of the induction study (or for patients with an SES-CD of 4 at baseline of the induction study, at least a 2-point reduction from baseline of the induction study). Endoscopic remission (secondary endpoint): SES-CD ≤4 and at least a 2-point reduction vs baseline and no subscore >1 in any individual variable.
UP NEXT
RINVOQ is an oral, once daily, selective and reversible JAK inhibitor now approved for the treatment of adult patients with moderately to severely active Crohn’s disease who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent.1
RINVOQ can be taken at any time of the day, with or without food. Tablets should be swallowed whole and should not be split, crushed, or chewed in order to ensure the entire dose is delivered correctly.1
A Phase 3 clinical trial program involving 3 studies: 2 12-week induction studies evaluated RINVOQ 45 mg QD vs placebo, and 1 52-week maintenance treatment and long-term extension study evaluated RINVOQ 15 mg QD and RINVOQ 30 mg QD vs placebo.1
Learn more about RINVOQ in our quick introductory video.
[Affiliates to insert local summary of safety]
REFERENCES
- RINVOQ [Summary of Product Characteristics]. AbbVie Deutschland GmbH & Co. KG; April 2023.
- Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570-1583. doi:10.1053/j.gastro.2020.12.031
- Loftus EV Jr, Colombel JF, Lacerda AP, et al. Efficacy and safety of upadacitinib induction therapy in patients with moderately to severely active Crohn’s disease: results from a randomized phase 3 U-EXCEL study. Presented at: United European Gastroenterology Week; October 8-11, 2022; Vienna, Austria.
- Data on file, AbbVie Inc. ABVRRTI75913.