RINVOQ SAFETY ANALYSES
from Phase 2 and 3 trials, and a head-to-head comparison trial in moderate to severe atopic dermatitis2,4,13
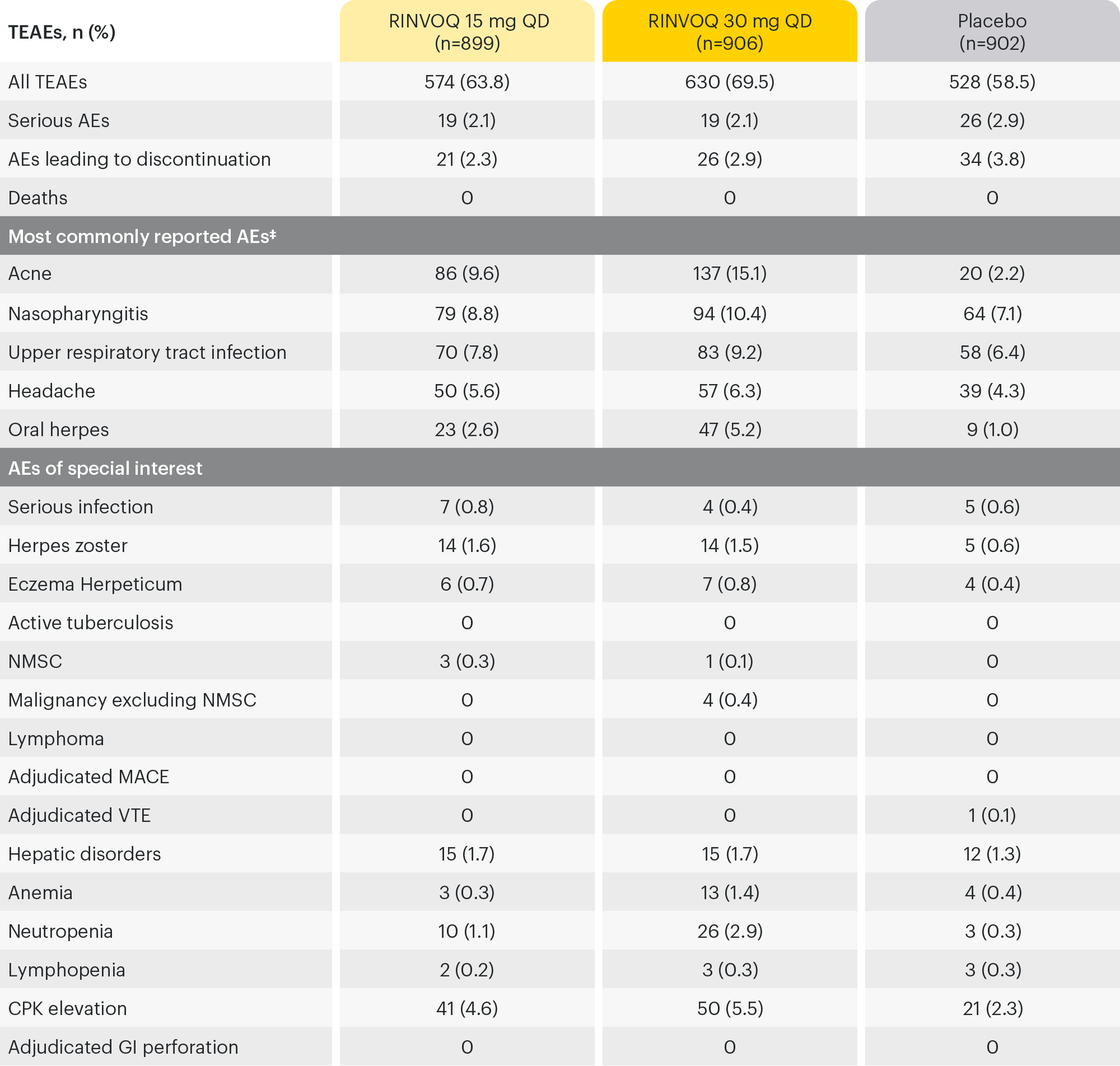
16-WEEK INTEGRATED SAFETY ANALYSIS (ALL SUBJECTS)1,13
The safety profile for RINVOQ 15 mg in adolescents was generally similar to that in adults.†
The safety profile of RINVOQ with long term treatment was generally similar to the safety profile during the placebo controlled period across indications.1
*Includes the randomized, placebo controlled Phase 2b study in adults ≥18 years with active moderate to severe AD, and the three pivotal Phase 3 studies in adults and adolescents ≥12 years with moderate to severe AD: MEASURE UP 1, MEASURE UP 2, and AD UP.13
**Use in adolescents is approved for 15 mg dosage option only. The safety and efficacy of RINVOQ in children with atopic dermatitis below the age of 12 years have not been established. No data are available. No clinical exposure data are available in adolescents <40 kg. The posology in adolescent patients 30 kg to <40 kg was determined using population pharmacokinetic modeling and simulation.1
†The safety and efficacy of the 30 mg dose in adolescents are still being investigated.
‡AEs reported for ≥5% of patients in any treatment group.
AE: adverse events; CPK: Creatine phosphokinase; GI: gastrointestinal; MACE: major adverse cardiac events; NMSC: mon-melanoma skin cancer; QD: once daily; TEAE: Treatment Emergent Adverse Event; VTE: venous thromboembolic events.
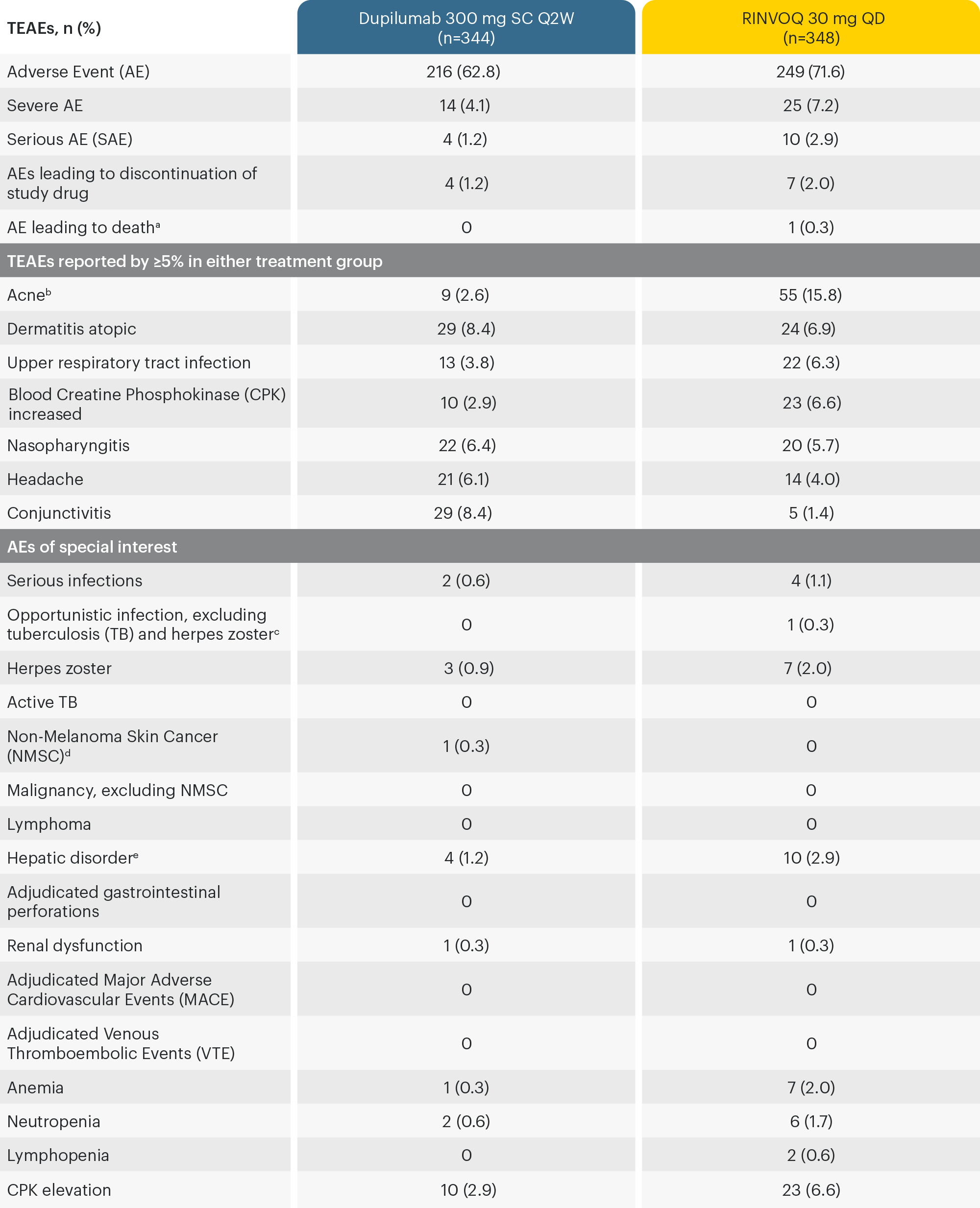
16-WEEK SAFETY ANALYSIS FROM HEADS UP TRIAL
*The safety and efficacy of the 30 mg dose in adolescents are still being investigated.
**Use in adolescents is approved for 15 mg dosage option only. The safety and efficacy of RINVOQ in children with atopic dermatitis below the age of 12 years have not been established. No data are available. No clinical exposure data are available in adolescents <40 kg. The posology in adolescent patients 30 kg to <40 kg was determined using population pharmacokinetic modeling and simulation.1
a40 year old woman found deceased at home on study Day 70 who had bronchopneumonia associated with influenza A; bMost acne events consisted primarily of inflammatory papules, pustules and comedones, involving the face. All events were non-serious. None led to treatment discontinuation; cAll opportunistic infections were eczema herpeticum; dKeratocanthoma; eHepatic disorders: most were elevated transaminases.4
TEAE: Treatment Emergent Adverse Event.
A well-studied safety profile with:
– >12,000* PATIENTS INVOLVED IN 18 PHASE 3 TRIALS IN THE RINVOQ CLINICAL TRIAL PROGRAMS ACROSS ALL INDICATIONS2,4,5,8-14
– APPROVED FOR AD PATIENTS ≥12 YEARS1
– 16 PHASE 3 TRIALS2,4,5,8–14
– 4 APPROVED INDICATIONS:1
– MODERATE TO SEVERE AD
– ACTIVE PSA
– ACTIVE AS
– MODERATE TO SEVERE ACTIVE RA
*10,500 patients includes all patients across all arms (active treatment and placebo) in the following Phase 3 trials in RA: (SELECT-EARLY, SELECT-NEXT, SELECT-COMPARE, SELECT-BEYOND, SELECT-CHOICE, SELECT-SUNRISE, SELECT-CHINA); PsA (SELECT-PsA, SELECT-PsA 2); AS (SELECT-AXIS 1); AD (RISING UP, MEASURE UP 1, MEASURE UP 2, AD UP, HEADS UP); UC (U-ACHIEVE, U-ACCOMPLISH). This includes 344 adolescent patients (aged 12 to 17 years) in the Phase 3 MEASURE UP 1, MEASURE UP 2 and AD UP studies in atopic dermatitis. Of the total number of patients included in these trials, 6,940 were randomized to receive RINVOQ at any dose (45 mg dosage for induction was included in UC studies). Study M16-048 included an upadacitinib 7.5 mg arm, which was not included in this analysis.
AD: atopic dermatitis; AS: ankylosing spondylitis; PsA: psoriatic arthritis; RA: rheumatoid arthritis.
Rheumatoid arthritis
RINVOQ is indicated for the treatment of moderate to severe active rheumatoid arthritis in adult patients who have responded inadequately to, or who are intolerant to one or more disease-modifying anti-rheumatic drugs (DMARDs). RINVOQ may be used as monotherapy or in combination with methotrexate.
AD: atopic dermatitis; AS: ankylosing spondylitis; PsA: psoriatic arthritis; RA: rheumatoid arthritis.
[Contraindications, special warnings and precautions, monitoring and lab abnormality disclaimers to be included here]
REFERENCES
- RINVOQ® (upadacitinib) Summary of Product Characteristics. AbbVie Deutschland GmbH & Co. KG: June 2022.
- Guttman-Yassky E, Teixeira H, Simpson E, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021; 397(10290): 2151–2168.
- Simpson EL, Papp KA, Blauvelt A, et al. Efficacy and safety of upadacitinib in patients with moderate to severe atopic dermatitis: analysis of follow-up data from the Measure Up 1 and Measure Up 2 randomized clinical trials. JAMA Dermatol. 2022;158(4):404–413.
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and Safety of Upadacitinib vs Dupilumab in Adults With Moderate-to-Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2021;157(9):1047–1055.
- European Medicines Agency. RINVOQ EPAR: Public Assessment Report. Available at: https://www.ema.europa.eu/en/medicines/human/ EPAR/rinvoq. Accessed April 2021.
- Cibinqo® (abrocitinib) Summary of Product Characteristics. Pfizer Limited. XXXX.
- Olumiant® (baricitinib) Summary of Product Characteristics. Eli Lilly and Company. XXXX.
- Reich K, Teixeira HD, Bruin-Weller, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021; 397(10290): 2169–2181.
- Rubbert-Roth A, Enejosa J, Pangan AL, et al. Trial of Upadacitinib or Abatacept in Rheumatoid Arthritis. N Engl J Med. 2020;383(16):1511–1521.
- Kameda H, Takeuchi T, Yamaoka K, et al. Efficacy and safety of upadacitinib in Japanese patients with rheumatoid arthritis (SELECT-SUNRISE): a placebo-controlled phase IIb/III study. Rheumatology (Oxford). 2020;59(11):3303–3313.
- Xiaofeng Zeng, Dongbao Zhao, Sebastiao Radominski. Efficacy and Safety of Upadacitinib in Patients From China, Brazil, and South Korea With Rheumatoid Arthritis Who Have Had Inadequate Response to Conventional Synthetic Disease-Modifying Antirheumatic Drugs. Abstract presented at the European Congress of Rheumatology, 3–6 June 2020. Abstract SAT0160.
- ClinicalTrials.gov. A Study to Evaluate Safety of Upadacitinib in Combination With Topical Corticosteroids in Adolescent and Adult Participants With Moderate to Severe Atopic Dermatitis (Rising Up). Available at: https://clinicaltrials.gov/ct2/show/NCT03661138 Accessed March 2021.
- Guttman-Yassky E, Irvine AD, Silverberg JI, et al. Upadacitinib in Moderate-to-Severe Atopic Dermatitis: Short-Term Safety in Phase 2b and Phase 3 Studies (M16-048, Measure Up 1, Measure Up 2, and AD Up). Poster presented at the American Academy of Dermatology (AAD) 2021 VMX Event. April 23-25, 2021. Poster 27082.
- Danese S, Vermeire S, et al. Lancet. 2022. [DRAFT MANUSCRIPT].