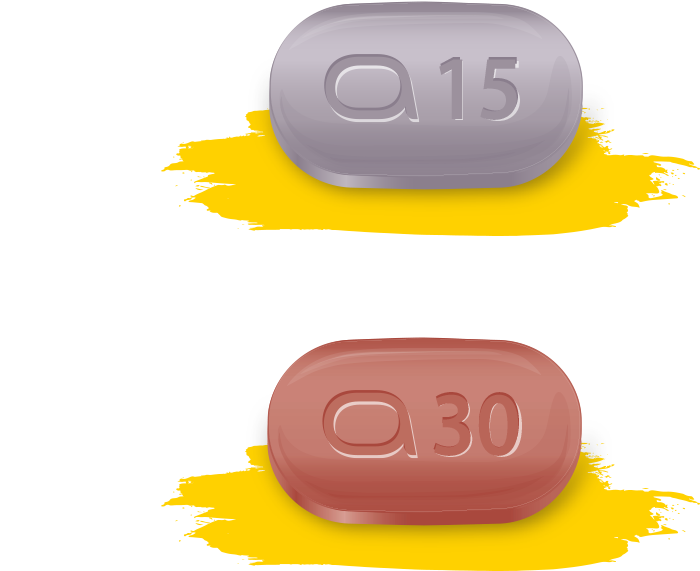
The recommended dose of RINVOQ is 15 mg or 30 mg once daily based on individual patient presentation.
– A dose of 30 mg once daily may be appropriate for patients with high disease burden
– A dose of 30 mg once daily may be appropriate for patients with an inadequate response to 15 mg once daily
– The lowest effective dose for maintenance should be considered
For patients ≥65 years of age, the recommended dose is 15 mg once daily.
For adolescents (from 12 to 17 years of age), the recommended dose is 15 mg once daily for adolescents weighing at least 30 kg.*
Consideration should be given to discontinuing RINVOQ treatment in any patient who shows no evidence of therapeutic benefit after 12 weeks of treatment.
*The safety and efficacy of RINVOQ in children with moderate to severe atopic dermatitis below the age of 12 years have not been established. No data are available. No clinical exposure data are available in adolescents <40 kg. The posology in adolescent patients 30 kg to <40 kg was determined using population pharmacokinetic modeling and simulation.1
REFERENCES
- RINVOQ® (upadacitinib) Summary of Product Characteristics. AbbVie Deutschland GmbH & Co. KG: June 2022.