See study designs, hear from experts, and download publications below.
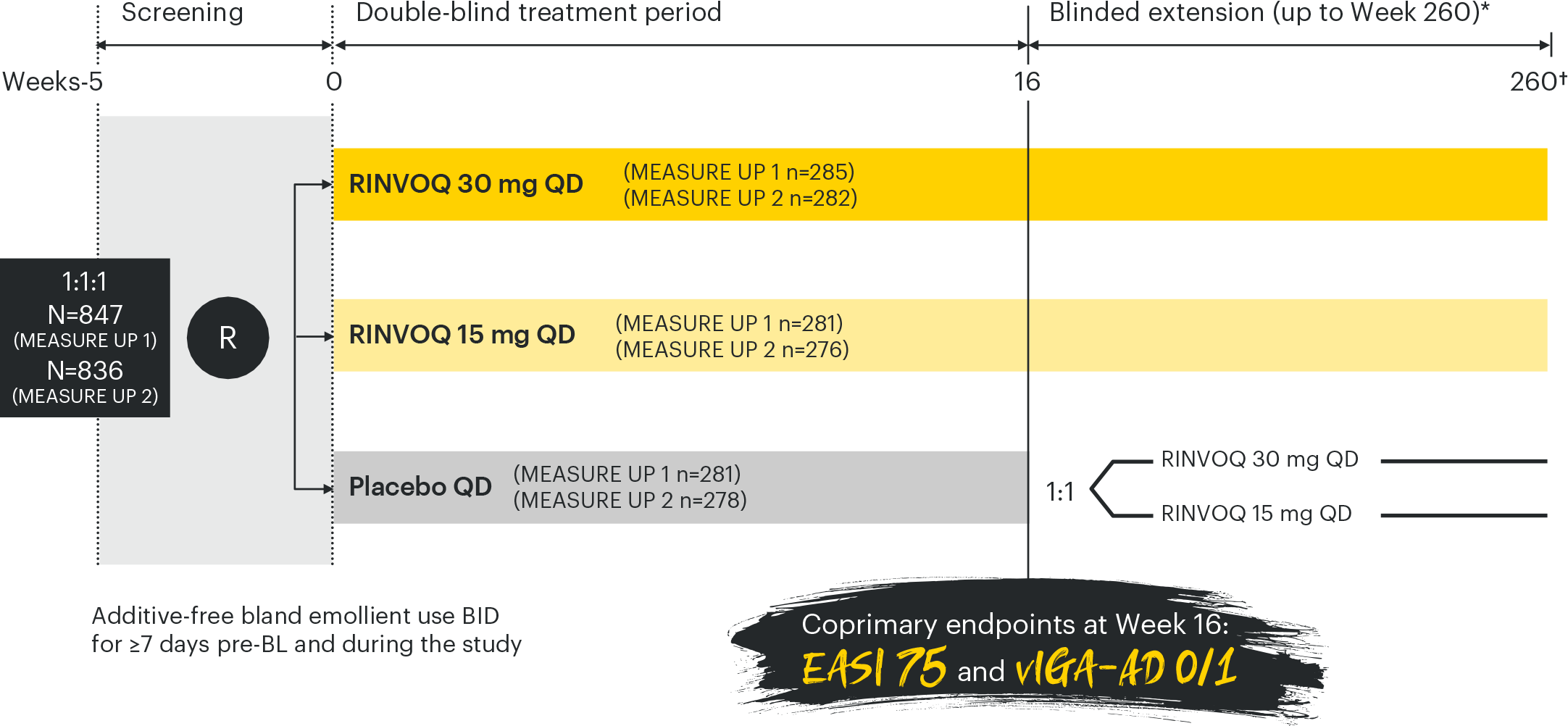
Efficacy analysis conducted in the ITT population of the double-blind treatment period and missing data for the co-primary and ranked secondary endpoints were handled using NRI-C (non-responder imputation incorporating MI to handle missing data due to COVID-19).2
*TCS were permitted during the blinded extension period and were not counted as rescue therapy.2 †30-day follow up.
AD: atopic dermatitis; BID: twice daily; BL: baseline; COVID-19: coronavirus disease 2019; EASI: Eczema Activity and Severity Index; ITT: intention-to-treat population; MI: multiple imputation; QD: once daily; TCS: topical corticosteroid; vIGA-AD 0/1: validated Investigator's Global Assessment for atopic dermatitis 0 or 1 response.
Key inclusion criteria2
– 12 to 75 yearsa with chronic ADb
– AD symptoms ≥3 years
– ≥10% BSA, EASI ≥16, and IGA ≥3
– BL weekly average of daily Worst Pruritus NRS ≥4
– Inadequate response to TCS or TCIc within 6 months prior to BL
Key exclusion criteria2
– Topical treatments within 7 days prior to BLd
– Systemic therapy for AD or phototherapye or traditional Chinese medicinef or any investigational drugg within 4 weeks prior to BL
– Prior exposure to dupilumab or systemic JAK inhibitors
aBody weight ≥40 kg at BL for subjects ≥12 and <18 yrs; bDiagnosis of AD according to the Hanifin and Rajka criteria (≥3 of 4 major features and ≥3 of 23 minor features); cor for patients for whom topical treatments were otherwise medically inadvisable; dexception of topical emollients; elaser therapy, tanning booth, or extended sun exposure that could affect disease severity or interfere with disease assessments; fOral or parenteral; gwithin 4 weeks or five half-lives of the drug (whichever is longer) or is currently enrolled in another clinical study.
AD: atopic dermatitis; BL: baseline; BSA: body surface area; EASI: eczema activity and severity index; IGA: investigator’s global assessment; JAK: Janus kinase; NRS: Numerical Rating Scale; TCI: topical calcineurin inhibitor; TCS: topical corticosteroids.
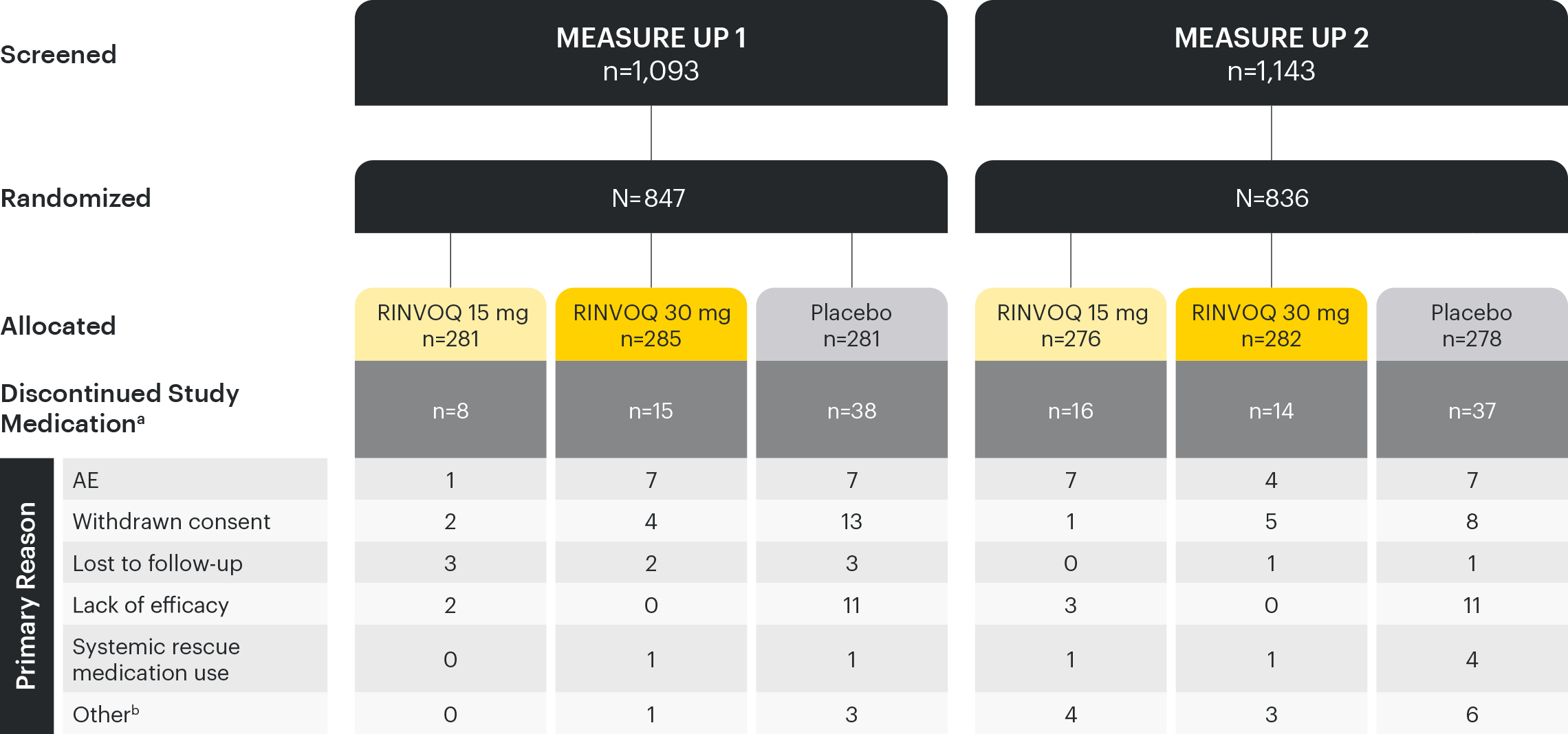
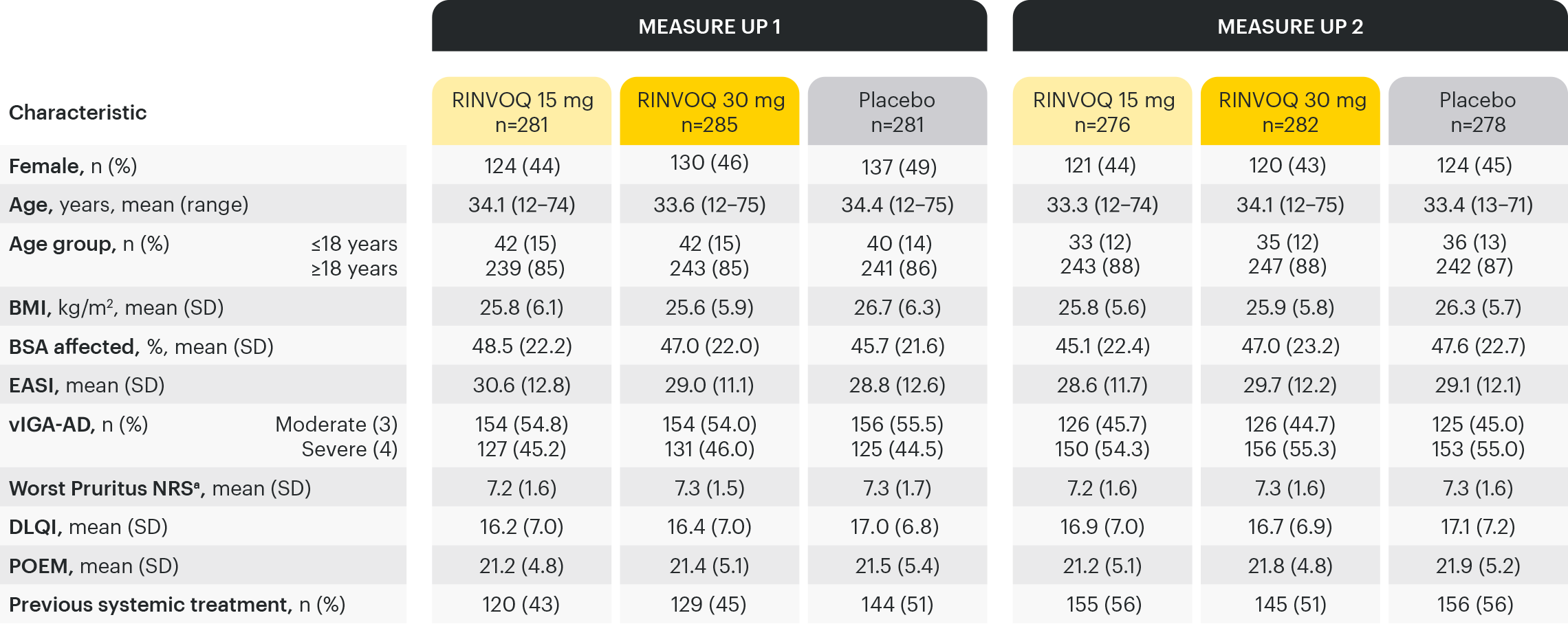
Based on ITT Population. Calculations are based on non-missing records.
aBased on weekly average.
AD: atopic dermatitis; BMI: body mass index; BSA: body surface area; DLQI: Dermatology Life Quality Index; EASI: Eczema Area and Severity Index; ITT: intent-to-treat for the main study; NRS: Numerical Rating Scale; POEM: Patient-oriented Eczema Measure; vIGA-AD: validated Investigator’s Global Assessment for AD.
REFERENCES
- RINVOQ® (upadacitinib) Summary of Product Characteristics. AbbVie Deutschland GmbH & Co. KG: June 2022.
- Guttman-Yassky E, Teixeira H, Simpson E, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021; 397(10290): 2151–2168.
- Reich K, Teixeira HD, Bruin-Weller, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021; 397(10290): 2169–2181.
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and Safety of Upadacitinib vs Dupilumab in Adults With Moderate-to-Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2021;157(9):1047–1055.