| * | Nothing on the skin: Defined as achievement of 75% PASI 90 and ≥84% sPGA 0/1 at Week 16 and achievement of ≥56% PASI 100 and sPGA 0 at Week 52 in UltIMMa-1 and UltIMMa-2.4 Please see full contextual information. |
NOTHING LESS THAN THE OPPORTUNITY FOR DURABLE SKIN CLEARANCE1,4
PIVOTAL TRIALS DATA1
In the UltIMMa-1 and -2 trials in adults with moderate to severe psoriasis, SKYRIZI patients met the co-primary endpoints of PASI 90 and sPGA of 0 or 1 at Week 16 vs placebo (NRI). UltIMMa-1 PASI 90 at W16: SKYRIZI 75.3% vs 4.9% placebo. UltIMMa-2 PASI 90 at W16: SKYRIZI 74.8% vs 2% placebo. UltIMMa-1 sPGA 0 or 1 at W16: SKYRIZI 87.8% vs 63% ustekinumab, 7.8% placebo. UltIMMa-2 sPGA 0 or 1 at W16: SKYRIZI 83.7% vs 5.1% placebo.
At Week 52, in UltIMMa-1 81.9% of SKYRIZI patients achieved PASI 90 vs 44% ustekinumab.
UltIMMa-2 PASI 90 at W52: SKYRIZI 80.6% vs 50.5% ustekinumab. All comparisons achieved P<0.001.
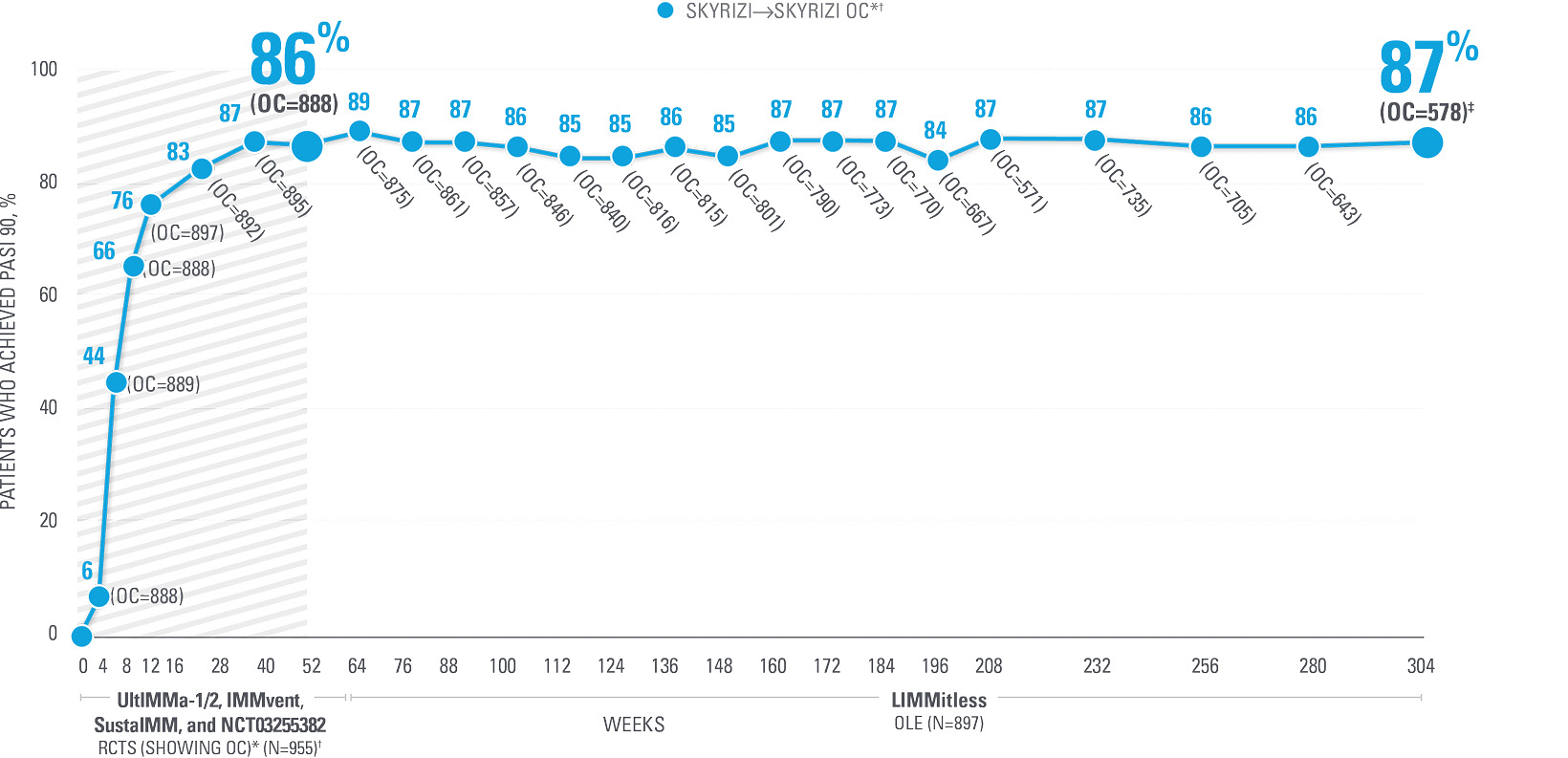
REVIEW PASI 90 DATA UP TO 5.5 YEARS
in patients with moderate to severe PsO from the LIMMitless open-label extension study5
Schedule a discussion with an AbbVie representative
[Affiliate To Update When They Localize]
| * | SKYRIZI dosing: Participants received 150 mg SKYRIZI at Week 0, Week 4, and every 12 weeks thereafter. | |
| Patients achieving PASI 90: Integrated UltlMMa-1/2, IMMvent, SustalMM, and NCT03255382 continuous SKYRIZI treatment data Week 0 to Week 304 (OC analysis).5 Results are not multiplicity controlled and no clinical or statistical conclusions can be drawn. | ||
| † | OC: Observed cases. No imputation of missing data; patients missing data at a visit were excluded from the observed analysis for that visit, which may increase the percent of responders as those who remain in the study generally fare better than those who discontinue. | |
| ‡ | Of the 671 patients who have either completed or are still ongoing in the LIMMitless study, 637 completed the assessment visit at Week 304; 59 ongoing patients have reached the assessment window but have not yet completed the assessment visit at Week 304.5 |
LIMMitless: Post-hoc subgroup analysis
In a post-hoc, subgroup analysis of patients in the LIMMitless Phase 3, single-arm, open-label extension study
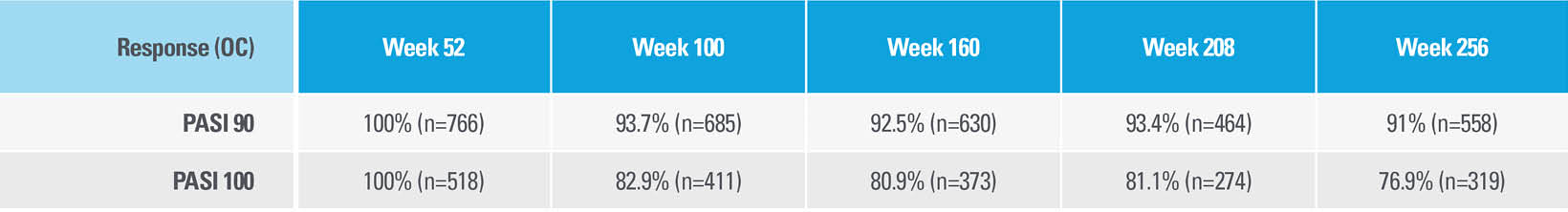
CONTINUATION OF DISEASE CONTROL WITH SKYRIZI WAS EVALUATED THROUGH 256 WEEKS6*†
LIMITATIONS:
- This analysis is post hoc, does not assess a prespecified endpoint, and was not adjusted for multiplicity. Therefore, no clinical or statistical conclusions can be drawn.
- For observed cases, there is no imputation of missing data; patients with missing data at a visit were excluded from the observed analysis for that visit. In an open-label extension with observed data, patients with missing data are excluded from the analysis and there is potential for enrichment of the long-term data as those who remain in the study generally fare better than those who discontinue.
Among patients who entered the LIMMitless Phase 3, single-arm, open-label extension study with PASI 90 at Week 52
>91% OF PATIENTS EVALUATED AT EACH TIME POINT CONTINUED TO DEMONSTRATE PASI 90 RESPONSE ON SKYRIZI
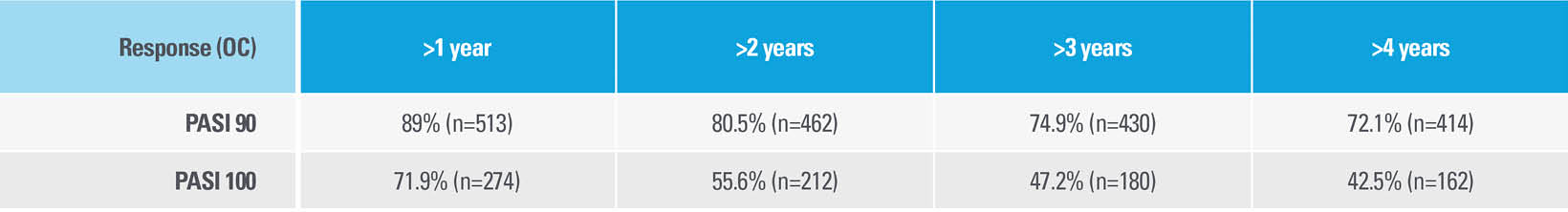
Among patients who entered the LIMMitless Phase 3, single-arm, open-label extension study with PASI 90 at Week 52
HIGH DISEASE CONTROL DEFINED AS NO LOSS OF PASI 90 WAS OBSERVED IN THE MAJORITY OF PATIENTS FOR OVER 4 YEARS†
| * | OC: Observed cases. No imputation of missing data; patients missing data at a visit were excluded from the observed analysis for that visit, which may increase the percent of responders. |
| † | High disease control was defined as no loss of PASI 90 or PASI 100, respectively, at any following visit once achieved in Year 1 and maintained for >84 days, for >1/>2/>3/>4 years. High disease control was assessed in patients who reached Year 4 and had <10% missing data of PASI. If a patient had a missing record at a visit but achieved the response before and after the visit, the patient was considered as no loss of response at that visit. |
| * | One non–treatment-emergent death of unknown cause on study Day 189 that occurred 161 days after the last dose of study drug. |
| † | One patient with sudden cardiac death on study Day 385 (101 days after last dose of study drug; event was not considered to be related to study drug by investigator). |
| ‡ | One patient with type 1 myocardial infarction on study Day 168 (event was not considered to be related to study drug by investigator). |
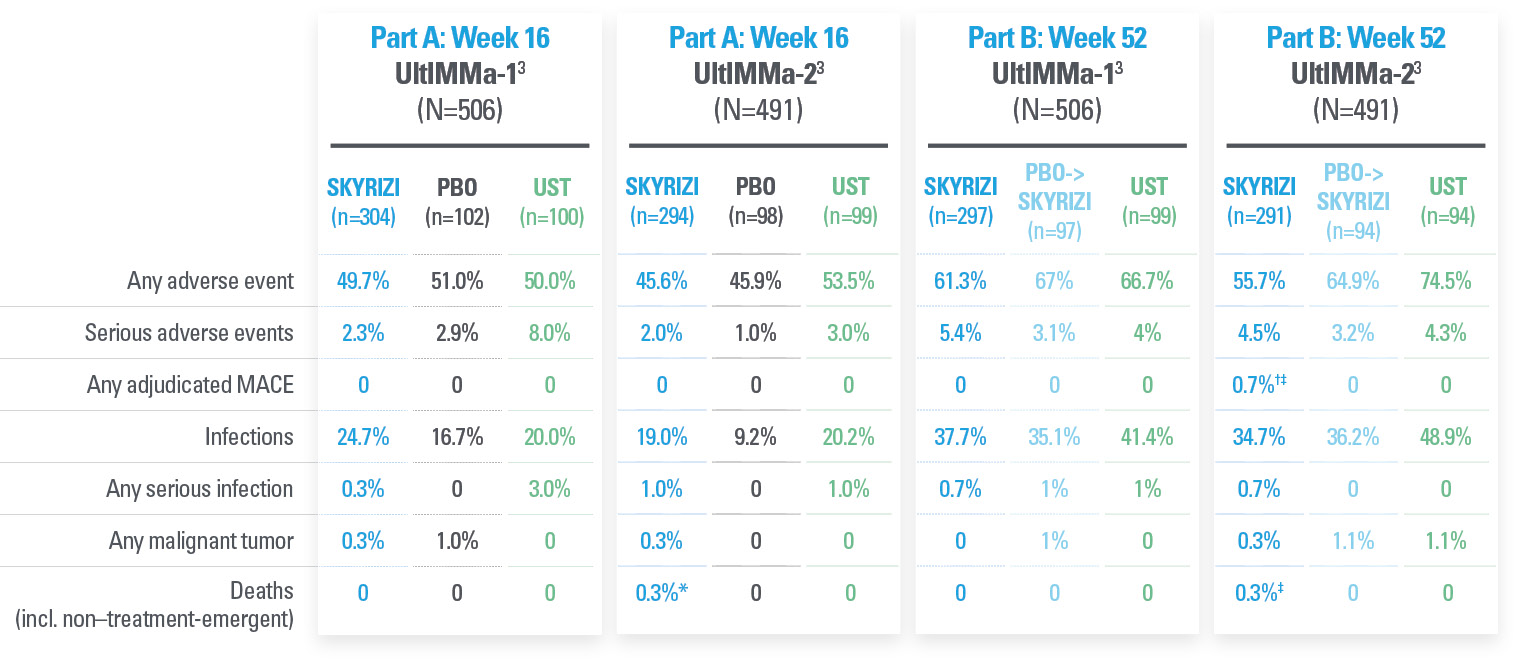
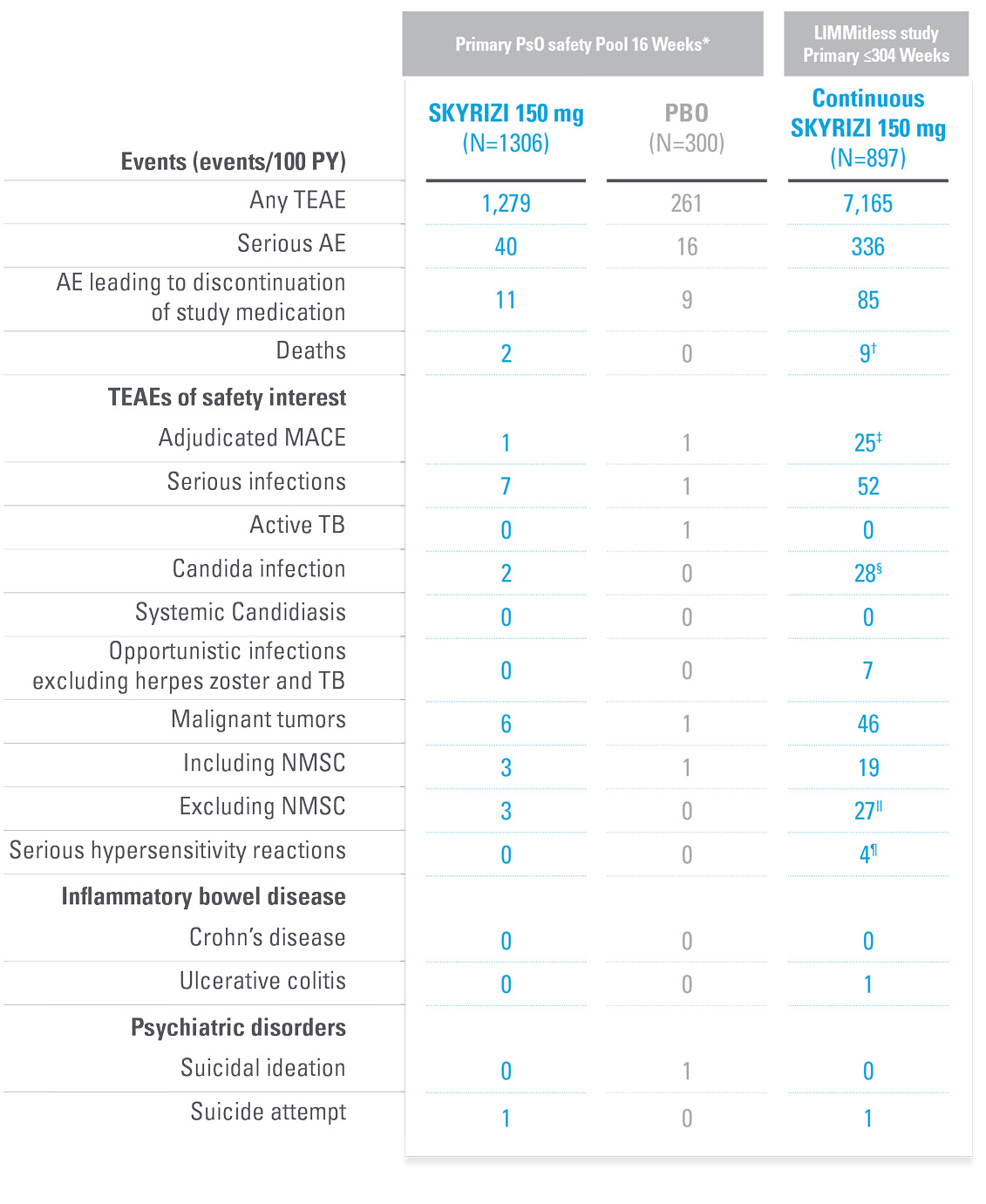
TREATMENT-EMERGENT ADVERSE EVENTS SUMMARY FROM LIMMitless5
| * | Primary PsO safety pool includes data from UltIMMa-1, UltMMa-2, IMMhance, and IMMvent, and NCT0205448120 studies. |
| † | Due to natural causes (n=1), accident (n=1), cardiopulmonary event (n=1), cardiac arrest (n=1), sudden cardiac death (n=1), cause unknown (n=3), COVID-19 infection (n=1); no deaths were related to study drug. |
| ‡ | MACE rate in the LIMMitless study is consistent with the incidence rate of MACE reported in the Psoriasis Longitudinal Assessment and Registry (PSOLAR; 0.57 E/100PY; 95% CI, 0.50–0.65). |
| § | Twenty-eight candida-related events were reported for 26 patients. More than half occurred in patients with known risk factors such as diabetes, steroid use, and prior candida episodes. None were serious or led to discontinuation of study drug. |
| ǁ | Malignancy types excluding NMSC were colorectal (n=7), skin (n=5), breast (n=4), prostate (n=3), urothelial (n=3), uterine (n=2), brain (n=1), gastric (n=1), and head and neck (n=1). |
| ¶ | Serious hypersensitivity reactions (all considered unrelated to study drug) were paraphenylenediamine allergy (n=1; mild, attributed to hair dye application), generalized microbial eczema (n=1; moderate, attributed to prolonged duration of generalized eczema and lack of response to treatment with hydrocortisone), Stevens-Johnson syndrome (n=2; severe, attributed to addition of chlorpromazine [n=1] and attributed to addition of Bactrim [n=1]). |
UltIMMa-1 and UltIMMa-2 Phase 3 study designs1,4
UltIMMa-1 (N=506) and UltIMMa-2 (N=491) were replicate Phase 3 multi-national, 52-week, randomized, double-blind, placebo-controlled, active comparator, controlled trials. Patients 18 years or older with moderate to severe plaque psoriasis were stratified by weight and previous exposure to TNF inhibitor and randomly assigned (3:1:1) to receive subcutaneous risankizumab 150 mg, ustekinumab 45 mg/90 mg (based on weight per label), or placebo.
Dosing occurred at Weeks 0 and 4 (during Part A) and Weeks 16, 28, and 40 (during Part B). Following the 16-week placebo-controlled treatment period (Part A), patients initially assigned to placebo switched to 150 mg of risankizumab at Week 16. Other patients continued double blind with their originally randomized treatment (Part B) for Weeks 16–52.
All efficacy analyses were done in the ITT population.
Co-primary endpoints: Proportion of patients achieving PASI 90 and an sPGA 0/1 at Week 16 (NRI).
LIMMitless OLE study design5
LIMMitless is an ongoing Phase 3, single-arm, multicenter, international, open-label extension (OLE) study evaluating the efficacy and safety of SKYRIZI (150 mg). All patients in LIMMitless received SKYRIZI 150 mg every 12 weeks for the duration of their participation in the study. Patients were initially randomized to receive SKYRIZI 150 mg in 1 of 5 Phase 2/3 studies: UltIMMa-1, UltIMMa-2, SustaIMM, IMMvent, and NCT03255382. 897 of 955 patients who completed 1 of the 5 base studies entered the LIMMitless OLE study and continued to receive SKYRIZI 150 mg every 12 weeks.
OLE limitations: In an open-label extension, there is a potential for enrichment of the long-term data in the remaining populations. Patients who are unable to tolerate or do not respond to the drug often drop out. Results are not multiplicity controlled and no clinical or statistical conclusions can be drawn.
Observed cases (OC): No imputation of missing data; patients missing data at a visit were excluded from the observed analysis for that visit.
Dosing: SKYRIZI 150 mg (two 75-mg subcutaneous injections) at Week 0, Week 4, and every 12 weeks thereafter.
EU INDICATIONS AND IMPORTANT SAFETY INFORMATION ABOUT SKYRIZI (risankizumab)
Indications1
Skyrizi (risankizumab) is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy.
Skyrizi, alone or in combination with methotrexate (MTX), is indicated for the treatment of active psoriatic arthritis in adults who have had an inadequate response or who have been intolerant to one or more disease-modifying antirheumatic drugs (DMARDs).
Skyrizi is indicated for the treatment of adult patients with moderately to severely active Crohn's disease who have had an inadequate response to, lost response to, or were intolerant to conventional therapy or a biologic therapy.
Important Safety Information1
Risankizumab is contraindicated in patients hypersensitive to the active substance or to any of the excipients, and in patients with clinically important active infections (e.g. active tuberculosis). Risankizumab may increase the risk of infection. In patients with a chronic infection, a history of recurrent infection, or known risk factors for infection, risankizumab should be used with caution. Treatment with risankizumab should not be initiated in patients with any clinically important active infection until the infection resolves or is adequately treated.
Patients treated with risankizumab should be instructed to seek medical advice if signs or symptoms of clinically important chronic or acute infection occur. If a patient develops such an infection or is not responding to standard therapy for the infection, the patient should be closely monitored and risankizumab should not be administered until the infection resolves.
Prior to initiating treatment with risankizumab, patients should be evaluated for tuberculosis (TB) infection. Patients receiving risankizumab should be monitored for signs and symptoms of active TB. Anti-TB therapy should be considered prior to initiating risankizumab in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed.
Prior to initiating therapy with risankizumab, completion of all appropriate immunisations should be considered according to current immunisation guidelines. If a patient has received live vaccination (viral or bacterial), it is recommended to wait at least 4 weeks prior to starting treatment with risankizumab. Patients treated with risankizumab should not receive live vaccines during treatment and for at least 21 weeks after treatment.
If a serious hypersensitivity reaction occurs, administration of risankizumab should be discontinued immediately and appropriate therapy initiated.
The most frequently reported adverse reactions were upper respiratory infections (from 13% in psoriasis to 15.6% in Crohn’s disease). Commonly (≥ 1/100 to < 1/10) reported adverse reactions included tinea infections, headache, pruritus, rash, fatigue, and injection site reactions.
This is not a complete summary of all safety information.
Please see the SmPC for complete prescribing information.
| References: 1. SKYRIZI [Summary of Product Characteristics]. AbbVie Ltd; September 2023. 2. Blome C, Gosau R, Radtke MA, et al. Patient-relevant treatment goals in psoriasis. Arch Dermatol Res. 2016;308(2):69-78. doi:10.1007/s00403-015-1613-8 3. Ryan C, Puig L, Zema C, et al. Incremental benefits on patient-reported outcomes for achieving PASI90 or PASI100 over PASI75 in patients with moderate to severe psoriasis. Poster presented at: 2018 European Academy of Dermatology and Venereology (EADV) Congress; September 12–16, 2018; Paris, France. Poster 2002. 4. Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392(10148):650-661. doi:10.1016/S0140-6736(18)31713-6 5. Papp K, Lebwohl MG, Puig L, et al. Long-term safety and efficacy of risankizumab for the treatment of moderate-to-severe plaque psoriasis: interim analysis from the LIMMitless open-label extension trial beyond 5.5 years of follow-up. Poster presented at: 2023 American Academy of Dermatology Annual Meeting (AAD), March 17-21, 2023; New Orleans, LA. 6. Warren RB, Lebwohl M, Costanzo A, et al. Long-term durability of efficacy, high disease control and state of remission of risankizumab in patients with moderate-to-severe psoriasis. Poster presented at: 2023 European Academy of Dermatology and Venereology (EADV) Congress; October 11-13, 2023. Berlin, Germany. |
| IL: interleukin; ITT: intent to treat; MACE: major adverse cardiovascular event; NRI: nonresponder imputation; OC: observed cases; OLE: open-label extension; PASI: Psoriasis Area and Severity Index; PBO: placebo; PsO: psoriasis; sPGA: static Physician’s Global Assessment; TNF: tumor necrosis factor; UST: ustekinumab. |
| This website is intended for [CountryName] Healthcare Professionals [Insert local AbbVie affiliate address] |
| © 2023 AbbVie. All rights reserved. |
| All trademarks are the property of their respective owners. No use of any AbbVie trademark, trade name, or trade dress in this site may be made without the prior written authorization of AbbVie Inc., except to identify the product or services of the company. |
| ALL-SKZD-230124 December 2023 |