Schedule a discussion with an AbbVie representative to discuss SKYRIZI PsA data
[Affiliate To Update When They Localize]
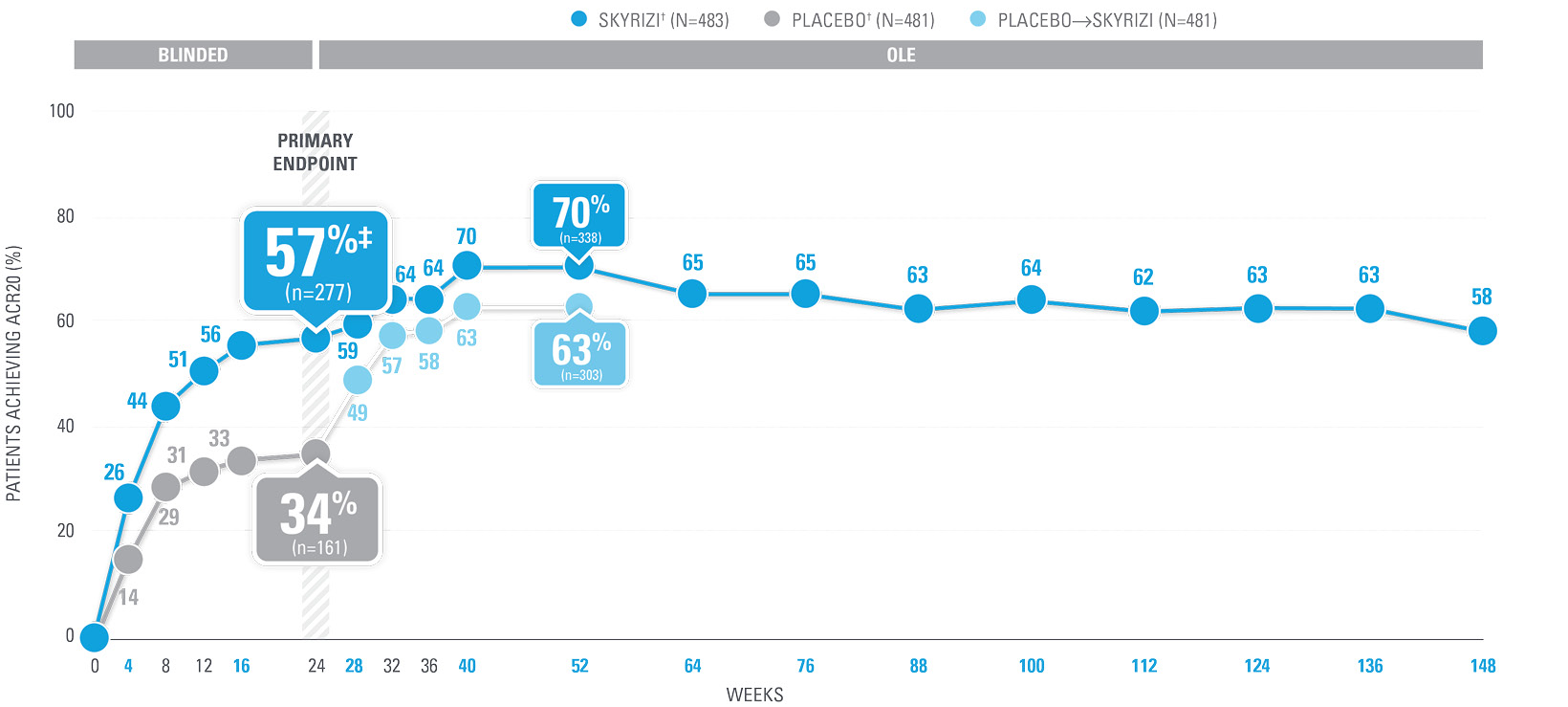
| SKYRIZI doses denoted in blue: Participants received 150 mg SKYRIZI at Week 0, Week 4, and every 12 weeks thereafter. Starting from Week 28, all subjects received SKYRIZI every 12 weeks. | |
| * | Summarized from KEEPsAKE-1 (bio-naïve population). |
| † | 65.5% of subjects from KEEPsAKE-1 taking placebo and 65% of SKYRIZI patients were receiving concomitant MTX. 10.2% of patients taking placebo and 10.8% of SKYRIZI patients were receiving concomitant nonbiologic DMARDs other than MTX.2 |
| ‡ | P≤0.001 vs placebo.1 |
| § | Weeks 52–100 were NRI-C and NRI-MI. Weeks 0–12 and 28–100 were not multiplicity controlled. |
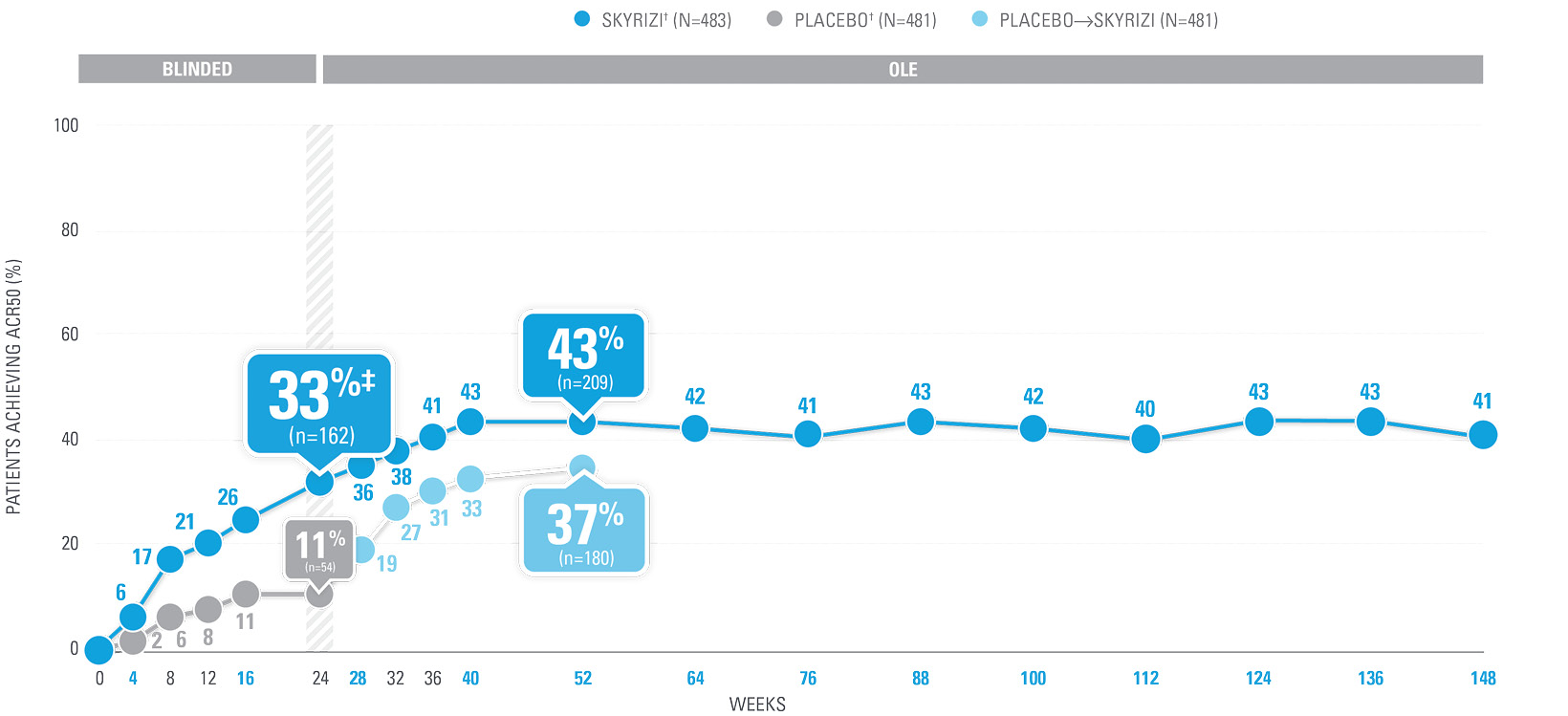
OVER 40% OF SKYRIZI PATIENTS ACHIEVED ACR50 AT WEEK 52 WITH CONSISTENT RATES THROUGH WEEK 100 (NRI)1-4*
| SKYRIZI doses denoted in blue: Participants received 150 mg SKYRIZI at Week 0, Week 4, and every 12 weeks thereafter. Starting from Week 28, all subjects received SKYRIZI every 12 weeks. | |
| ACR50 was a prespecified, non-ranked secondary endpoint. | |
| * | Summarized from KEEPsAKE-1 (bio-naïve population). |
| † | 65.5% of subjects from KEEPsAKE-1 taking placebo and 65% of SKYRIZI patients were receiving concomitant MTX. 10.2% of patients taking placebo and 10.8% of SKYRIZI patients were receiving concomitant nonbiologic DMARDs other than MTX.2 |
| ‡ | Nominal P≤0.001 vs placebo, not multiplicity-controlled.1 |
| § | Weeks 52–100 were NRI-C and NRI-MI. |
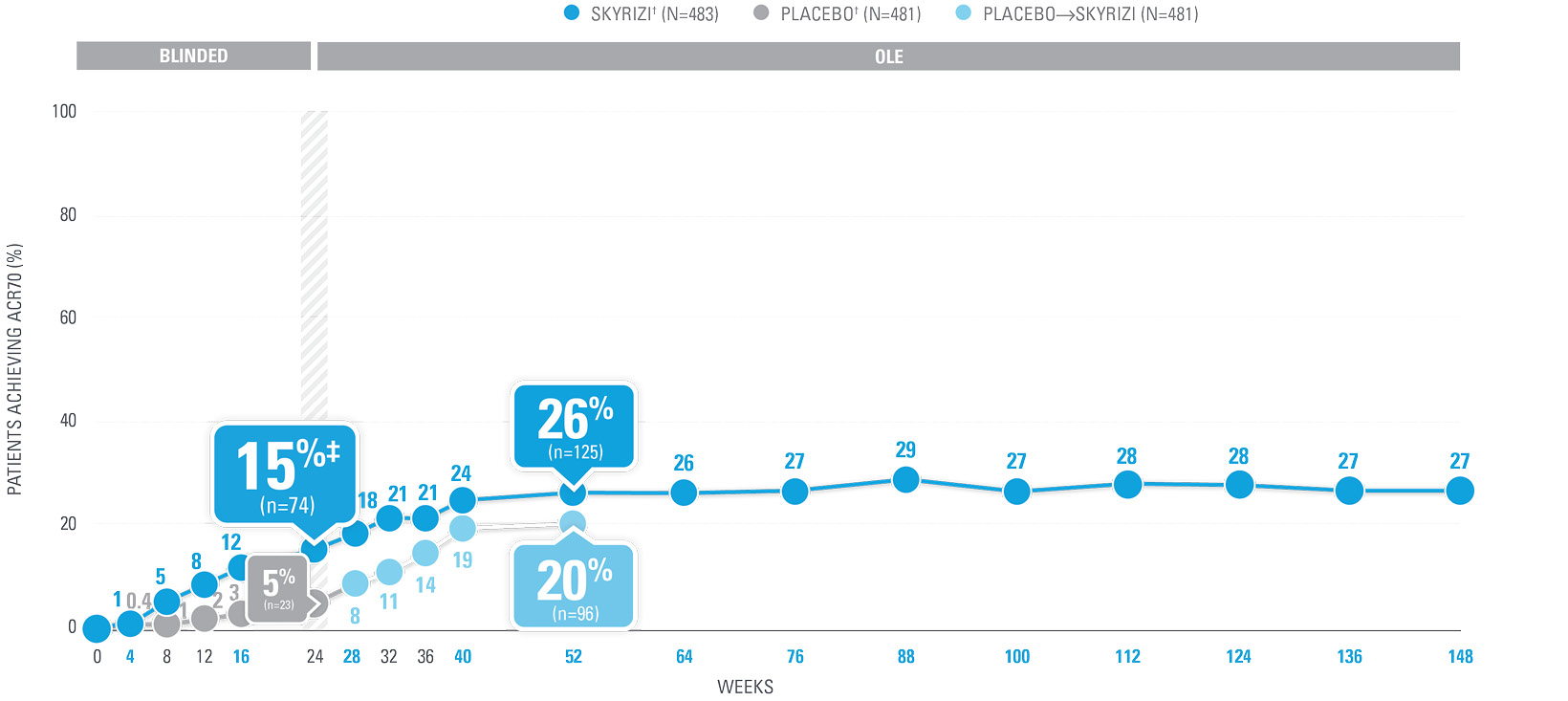
ACR70 RESPONSE RATES with SKYRIZI THROUGH WEEK 100 (NRI)1-4*
| SKYRIZI doses denoted in blue: Participants received 150 mg SKYRIZI at Week 0, Week 4, and every 12 weeks thereafter. Starting from Week 28, all subjects received SKYRIZI every 12 weeks. | |
| ACR70 was a prespecified, non-ranked secondary endpoint. | |
| * | Summarized from KEEPsAKE-1 (bio-naïve population). |
| † | 65.5% of subjects from KEEPsAKE-1 taking placebo and 65% of SKYRIZI patients were receiving concomitant MTX. 10.2% of patients taking placebo and 10.8% of SKYRIZI patients were receiving concomitant nonbiologic DMARDs other than MTX.2 |
| ‡ | Nominal P≤0.001 vs placebo, not multiplicity-controlled.1 |
| § | Weeks 52–100 were NRI-C and NRI-MI. |
| Observed cases (OC): No imputation of missing data; patients missing data at a visit were excluded from the observed analysis for that visit, which may increase the percent of responders. | |
| Percentage reduction was calculated by dividing the mean change at Week 52 (27.5) by the baseline score (57.1). Assessment is based on the Visual Analog Scale (100 mm), with the left end indicating “no pain” (for patient assessment of pain), “very well” (for patient global assessment), or “no arthritis activity” (for physician global assessment) and the right end indicating “the worst possible pain” (for patient assessment of pain), “poor” (for patient global assessment), or “extremely active arthritis” (for physician global assessment). | |
| * | 59.6% of subjects from both KEEPsAKE-1 and KEEPsAKE-2 studies were receiving concomitant MTX, 11.6% were receiving concomitant nonbiologic DMARDs other than MTX, and 28.9% were receiving SKYRIZI monotherapy.1 |
| Patient assessment of pain is one of the 7 core set measures to determine ACR for patients with PsA. The results of SKYRIZI 150 mg at Week 52 for the remaining core measures in KEEPsAKE-1 (AO) were as follows:5 | |
| • Swollen joint count (N=444) mean baseline was 12.1, with a change of -10.5 [-11.1, -9.8] • Tender joint count (N=444) mean baseline was 20.8, with a change of -16.1 [-17.2, -14.9] • Physician assessment of disease activity (N=419) mean baseline was 61.4, with a change of -45.1 [-47.3, -43.0] • Patient’s assessment of disease activity (N=441) baseline was 57.8, with a change of -28.6 [-31.2, -25.9] • Health Assessment Questionnaire Disability Index (HAQ-DI) (N=441) baseline was 1.16, with a change of -0.43 [-0.48, -0.37] • CRP levels (N=438) baseline was 11.44, with a change of -4.39 [-5.63, -3.15]. | |
| All data listed with a 95% CI. |
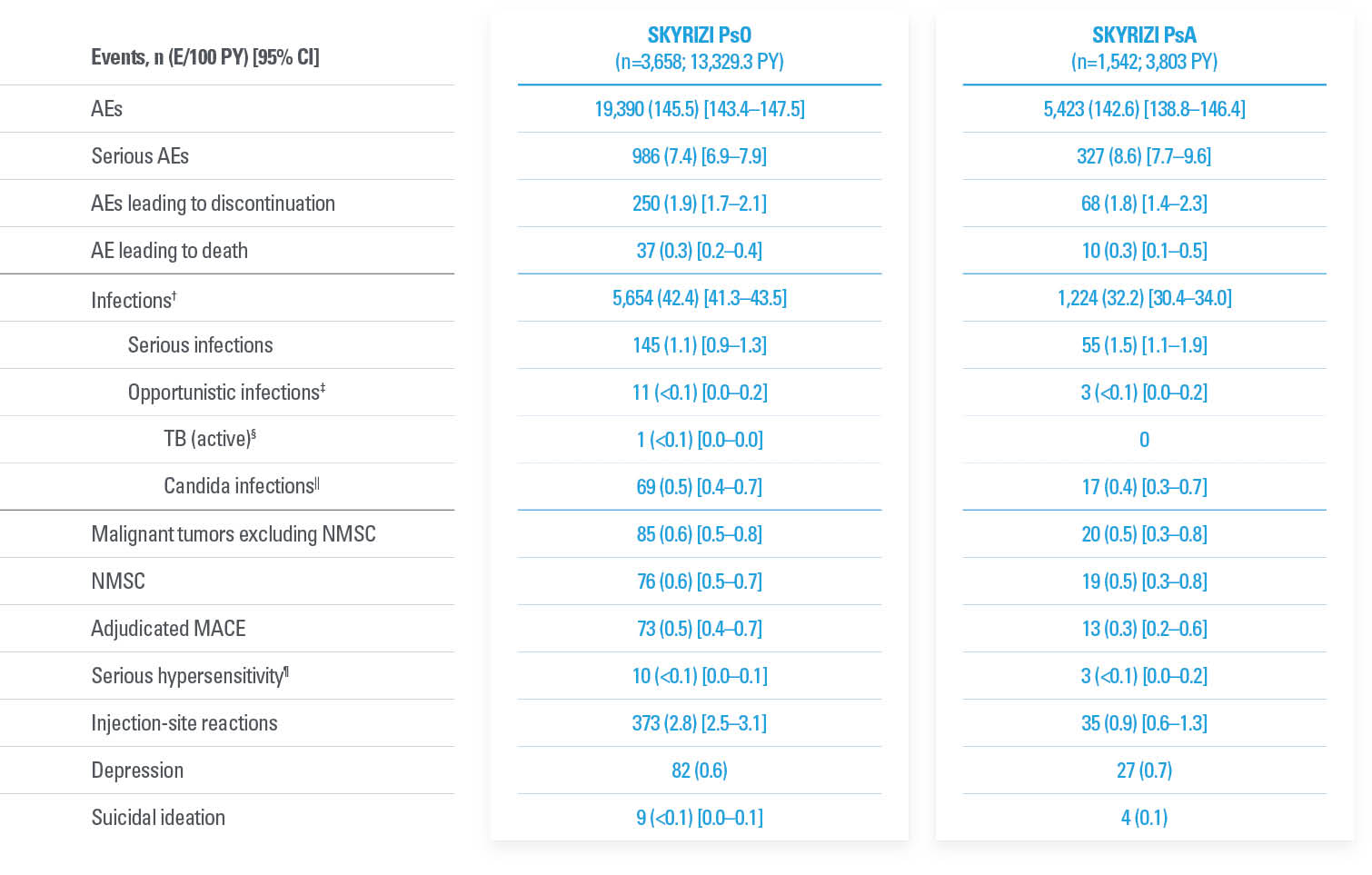
PsA SAFETY PROFILE CONSISTENT WITH PsO
No new safety signals observed1
| * | KEEPsAKE-1: Except for pneumonia, which was reported for 2 patients (0.4%) in the placebo group, no serious AE or severe TEAE was reported for >1 patient in either group. |
| † | One death (urosepsis) in an 81-year-old male patient. |
| ‡ | KEEPsAKE-1 SKYRIZI: urosepsis (1 patient, resulting in death), cellulitis (1 patient), gastroenteritis (1 patient), COVID-19 pneumonia (1 patient), and viral upper respiratory tract infection leading to pneumonia (1 patient); placebo: pneumonia (2 patients), oral bacterial infection (one patient), dysentery (1 patient), appendicitis (1 patient), and cellulitis (1 patient). KEEPsAKE-2 SKYRIZI: abscess and cellulitis (1 patient) and gastroenteritis (1 patient); placebo: erysipelas, gastroenteritis, postoperative abscess, upper respiratory tract infection, and urinary tract infection (each reported for 1 patient). |
| § | KEEPsAKE-1: All nonserious, resolved with oral antiviral agents and did not result in discontinuation of the study drug. |
| || | KEEPsAKE-2: Both were nonmelanoma skin cancer. |
| ¶ | All nonserious and did not result in discontinuation of the study drug. |
FAVORABLE SAFETY PROFILE IN PsO AND PsA7,8
Evaluated up to 8.8 years in PsO and up to 4 years in PsA7
Treatment-emergent AEs from an integrated analysis of PsO and PsA clinical trials7*
| * | Long-term safety was evaluated using integrated all-risankizumab safety data sets (data cutoff March 25, 2023) from 20 Phase 1–4 clinical trials in PsO and 4 Phase 2 and 3 trials in PsA. Median (range) of treatment duration for PsO was 4.1 years (81 days to 8.8 years) and for PsA was 2.8 years (84 days to 4.0 years). For all patients who received ≥1 dose of risankizumab (all administered doses, 18 mg to 180 mg), AEs and AEs of special interest were assessed and recorded through the end of exposure (last dose to first dose + 5 geometric-mean half-lives [20 weeks]). |
| † | Excluding COVID-related infections. |
| ‡ | Excluding tuberculosis and herpes zoster. |
| § | One case of active tuberculosis was reported from Taiwan. The patient in a long-term open-label psoriasis study had latent tuberculosis diagnosed at screening of the feeder study and received isoniazid prophylaxis. He presented with a cough for 4 years after study initiation and was diagnosed based on positive sputum and chest x-ray (diagnostic results such as PCR/culture were not provided). |
| || | By system organ class. |
| ¶ | Reported events: eczema (2), Stevens-Johnson syndrome (2), urticaria (2), angioedema (1), drug hypersensitivity (1), erythema multiforme (1), and hypersensitivity (1). |
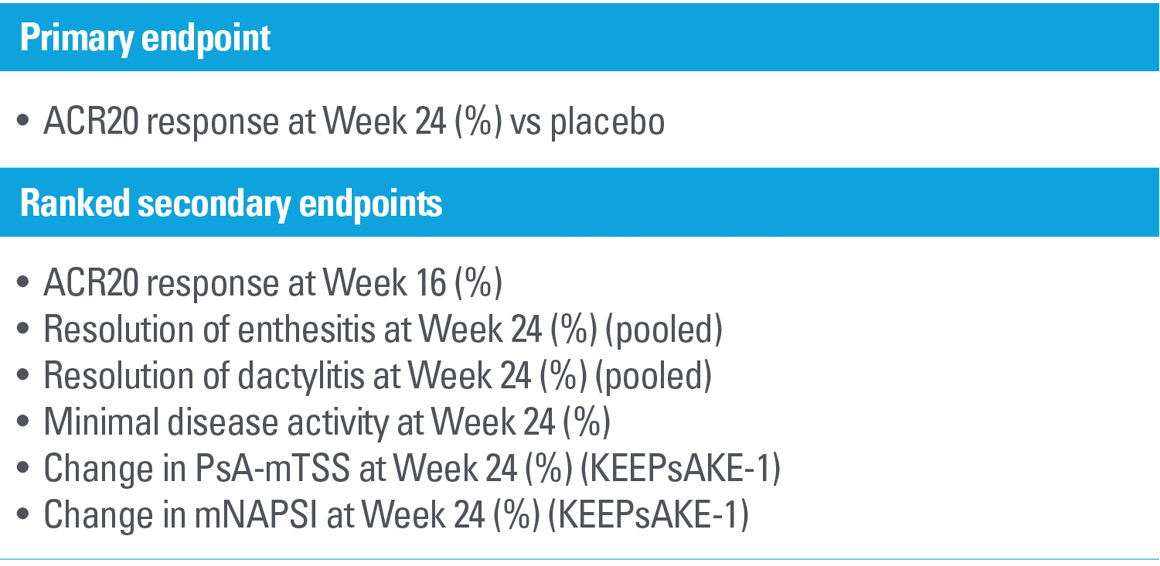
Study design
KEEPsAKE-1 and KEEPsAKE-21,2,6
Two randomized, double-blind, placebo-controlled studies assessing the safety and efficacy of 1,407 patients (964 in KEEPsAKE-1 and 443 in KEEPsAKE-2) ≥18 years old with active PsA.
Patients had a diagnosis of PsA ≥6 months based on Classification Criteria for Psoriatic Arthritis (CASPAR), a median duration of PsA of 4.9 years at baseline, ≥5 tender joints and ≥5 swollen joints, and active plaque psoriasis or nail psoriasis at baseline. 55.9% of subjects had ≥3% body surface area with active plaque psoriasis.
63.4% and 27.9% of subjects had enthesitis and dactylitis, respectively. In KEEPsAKE-1, all subjects had a previous inadequate response or intolerance to nonbiologic DMARD therapy and were biologic naïve. In KEEPsAKE-2, 53.5% of subjects had a previous inadequate response or intolerance to nonbiologic DMARD therapy, and 46.5% of subjects had a previous inadequate response or intolerance to biologic therapy.
In both studies, subjects were randomized to receive SKYRIZI 150 mg or placebo at Weeks 0, 4, and 16. Starting from Week 28, all subjects received SKYRIZI every 12 weeks. Both studies include a long-term extension for up to an additional 204 weeks. 59.6% of subjects from both studies were receiving concomitant MTX, 11.6% were receiving concomitant nonbiologic DMARDs other than MTX, and 28.9% were receiving SKYRIZI monotherapy.
Dosing: SKYRIZI was dosed 150 mg (two 75-mg subcutaneous injections) at Week 0, Week 4, and every 12 weeks thereafter.
EU INDICATIONS AND IMPORTANT SAFETY INFORMATION ABOUT SKYRIZI (risankizumab)
Indications1
Skyrizi (risankizumab) is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy.
Skyrizi, alone or in combination with methotrexate (MTX), is indicated for the treatment of active psoriatic arthritis in adults who have had an inadequate response or who have been intolerant to one or more disease-modifying antirheumatic drugs (DMARDs).
Skyrizi is indicated for the treatment of adult patients with moderately to severely active Crohn's disease who have had an inadequate response to, lost response to, or were intolerant to conventional therapy or a biologic therapy.
Important Safety Information1
Risankizumab is contraindicated in patients hypersensitive to the active substance or to any of the excipients, and in patients with clinically important active infections (e.g. active tuberculosis). Risankizumab may increase the risk of infection. In patients with a chronic infection, a history of recurrent infection, or known risk factors for infection, risankizumab should be used with caution. Treatment with risankizumab should not be initiated in patients with any clinically important active infection until the infection resolves or is adequately treated.
Patients treated with risankizumab should be instructed to seek medical advice if signs or symptoms of clinically important chronic or acute infection occur. If a patient develops such an infection or is not responding to standard therapy for the infection, the patient should be closely monitored and risankizumab should not be administered until the infection resolves.
Prior to initiating treatment with risankizumab, patients should be evaluated for tuberculosis (TB) infection. Patients receiving risankizumab should be monitored for signs and symptoms of active TB. Anti-TB therapy should be considered prior to initiating risankizumab in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed.
Prior to initiating therapy with risankizumab, completion of all appropriate immunisations should be considered according to current immunisation guidelines. If a patient has received live vaccination (viral or bacterial), it is recommended to wait at least 4 weeks prior to starting treatment with risankizumab. Patients treated with risankizumab should not receive live vaccines during treatment and for at least 21 weeks after treatment.
If a serious hypersensitivity reaction occurs, administration of risankizumab should be discontinued immediately and appropriate therapy initiated.
The most frequently reported adverse reactions were upper respiratory infections (from 13% in psoriasis to 15.6% in Crohn’s disease). Commonly (≥ 1/100 to < 1/10) reported adverse reactions included tinea infections, headache, pruritus, rash, fatigue, and injection site reactions.
This is not a complete summary of all safety information.
Please see the SmPC for complete prescribing information.
| References: 1. SKYRIZI [Summary of Product Characteristics]. AbbVie Ltd; September 2023. 2. Kristensen LE, Keiserman M, Papp K, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 1 trial. Ann Rheum Dis. 2022;81(2):225-231. doi:10.1136/annrheumdis-2021-221019 3. Kristensen L, Papp K, White D, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 100-week results from the KEEPsAKE 1 and KEEPsAKE 2 trials. Poster presented at: 2022 European Academy of Dermatology and Venereology (EADV) Congress; September 7-10, 2022; Milan, Italy and Virtual 4. Kristensen LE, Keiserman M, Papp K, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 52-week results from the KEEPsAKE 1 study. Rheumatology (Oxford). 2023;62(6):2113-2121. doi:10.1093/rheumatology/keac607 5. Data on File, AbbVie Inc. ABVRRTI74973. 6. Östör A, Van den Bosch F, Papp K, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 2 trial. Ann Rheum Dis. 2022;81(3):351-358. doi:10.1136/annrheumdis-2021-221048 7. Gordon KB, Blauvelt A, Bachelez H, et al. Long-term safety of risankizumab in patients with psoriatic disease: integrated analysis of psoriasis and psoriatic arthritis clinical trial data. Poster presented at: 2023 European Academy of Dermatology and Venereology (EADV) Congress; October 11-14, 2023; Berlin, Germany. 8. Gordon KB, Blauvelt A, Coates L, et al. Risankizumab long-term safety in patients with psoriatic disease: integrated analyses of data from psoriasis and psoriatic arthritis clinical trials. Poster presented at: 2022 European Academy of Dermatology and Venereology (EADV) Virtual Congress; September 7-10, 2022 ; Milan, Italy Poster 1607. |
| This website is intended for [CountryName] Healthcare Professionals |
| [Insert local AbbVie affiliate address] |
| © 2023 AbbVie. All rights reserved. |
| All trademarks are the property of their respective owners. No use of any AbbVie trademark, trade name, or trade dress in this site may be made without the prior written authorization of AbbVie Inc., except to identify the product or services of the company. |
| ALL-SKZD-230125 December 2023 |