LUMIGAN® (bimatoprost ophthalmic solution)
LUMIGAN® 0.03% UD is indicated for the reduction of elevated intraocular pressure in chronic open-angle glaucoma and ocular hypertension (as monotherapy or as adjunctive therapy to beta-blockers):1
LUMIGAN® (bimatoprost ophthalmic solution 0.01%)
LUMIGAN® (bimatoprost ophthalmic solution 0.03% UD)
What is the efficacy of LUMIGAN® 0.03% UD?
LUMIGAN® 0.03% UD can offer effective IOP-lowering with
once-daily dosing1–3
LUMIGAN® 0.03% UD achieved equivalent IOP reduction to LUMIGAN® 0.03% MD in worse eye IOP at every timepoint over 12 weeks.*,1,3
*Results of a double-masked, parallel-group study of 597 patients (with OHT and/or chronic open-angle glaucoma, chronic-angle closure glaucoma with patent iridotomy/iridectomy, pseudoexfoliative glaucoma or pigmentary glaucoma) who were randomized to LUMIGAN® 0.03% UD (n=302) or LUMIGAN® 0.03% MD (n=295) for 12 weeks, with analysis for non-inferiority in change from baseline in worse eye IOP conducted at weeks 2, 6, and 12. Adverse events were reported for 40.5% (n=122/301) of LUMIGAN® 0.03% UD and 44.1% (n=130/295) of LUMIGAN® 0.03% MD patients (p=0.382), with ocular adverse events reported for 31.9% and 34.9%, respectively (p=0.434). The most frequent adverse event was conjunctival hyperemia, considered to be treatment-related in all except one patient in each group, and reported as mild or moderate in the majority of patients.3
Mean average IOP at each time point, for patients with glaucoma or OHT treated with either LUMIGAN® 0.03% UD or LUMIGAN® 0.03% MD, N=5973
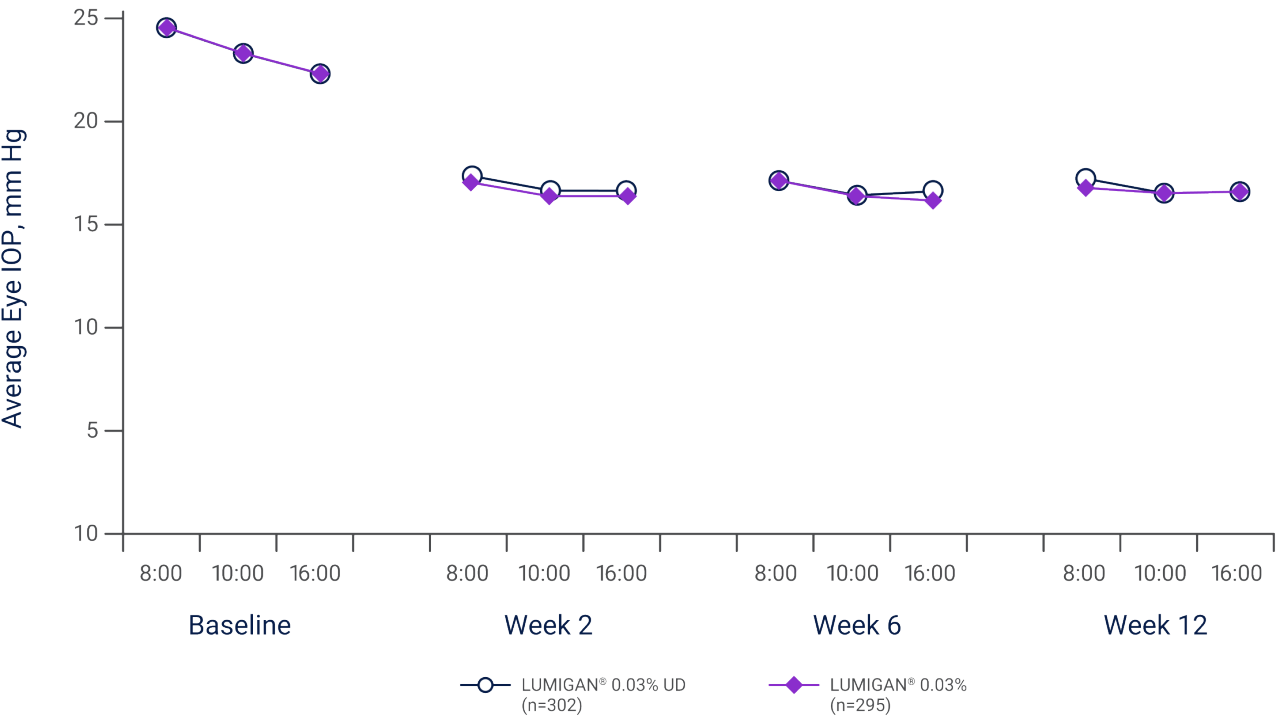
Adapted from Day D et al. 2013.3
Results of a double-masked, parallel-group study of 597 patients (with OHT and/or chronic open-angle glaucoma, chronic-angle closure glaucoma with patent iridotomy/iridectomy, pseudoexfoliative glaucoma or pigmentary glaucoma) who were randomized to LUMIGAN® 0.03% UD (n=302) or LUMIGAN® 0.03% MD (n=295) for 12 weeks, with analysis for non-inferiority in change from baseline in worse eye IOP conducted at weeks 2, 6, and 12. Mean change from baseline ranged from -7.49 mmHg to -5.93 mmHg for LUMIGAN® 0.03% UD and -7.77 mmHg to -6.06 mmHg for LUMIGAN® 0.03% MD. Adverse events were reported for 40.5% (n=122/301) of LUMIGAN® 0.03% UD and 44.1% (n=130/295) of LUMIGAN® 0.03% MD patients (p=0.382), with ocular adverse events reported for 31.9% and 34.9%, respectively (p=0.434). The most frequent adverse event was conjunctival hyperemia, considered to be treatment-related in all except one patient in each group, and reported as mild or moderate in the majority of patients.3
IOP, intraocular pressure; MD, multi dose; OHT, ocular hypertension; UD, unit dose.
Based on a real world study, patients with POAG or OHT who switched to LUMIGAN® 0.03% UD demonstrated mean IOP reductions from baseline of 23% (21.64 mmHg to 16.59 mmHg, p<0.0001; n=1543).**,2
**Results of an open-label study evaluating the efficacy and tolerability of, and compliance to, LUMIGAN® 0.03% UD in 1,830 patients with POAG and OHT who were switched to LUMIGAN® 0.03% UD from previously prescribed topical IOP-lowering therapy due to medical reasons. AEs were experienced by 5.7% of patients, and there were no serious AEs reported. The most common AEs were eye irritation (1.7%) and hyperemia (1.4%).2
Real-world evidence is collected outside of controlled clinical trials and has inherent limitations including a lesser ability to control for confounding factors.
Overall mean±SD IOP in patients who switched from a prior IOP-lowering therapy to LUMIGAN® 0.03% UD2
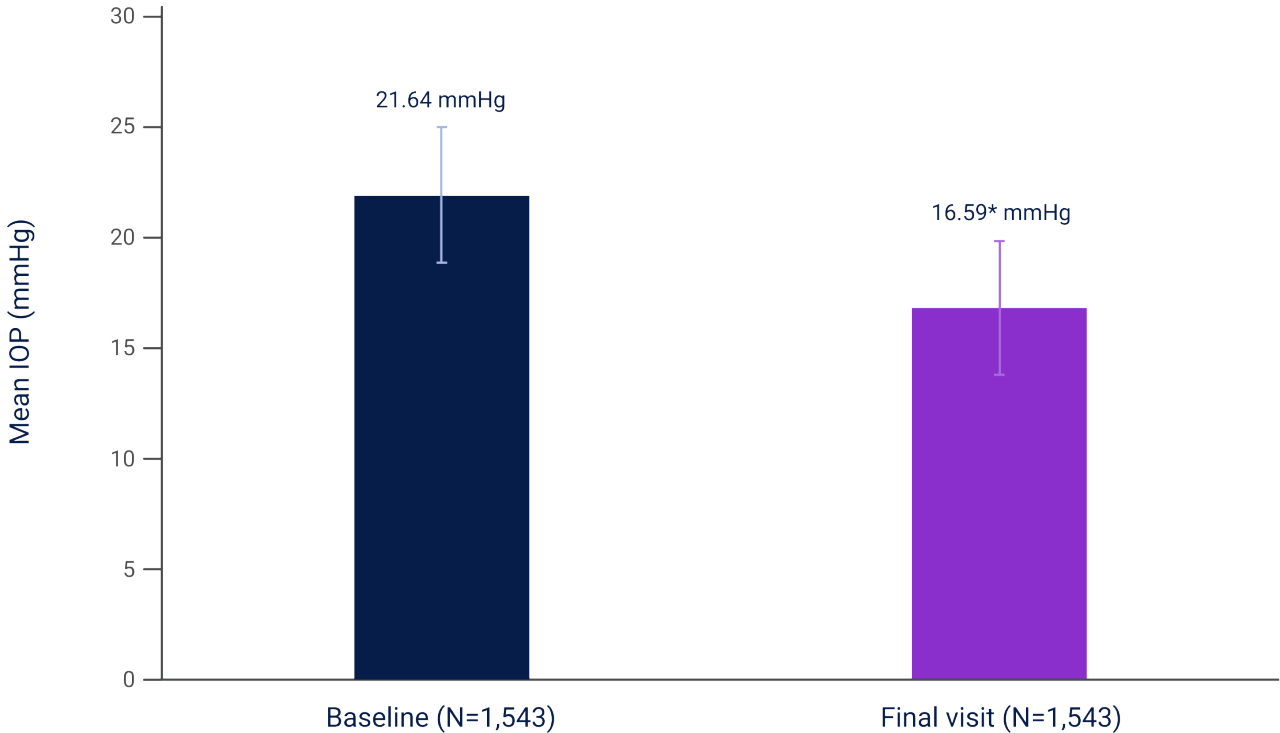
Adapted from Pillunat L et al. 2016.2
Results of an open-label study evaluating the efficacy and tolerability of, and compliance to, LUMIGAN® 0.03% UD in 1,830 patients with POAG or OHT who were switched to LUMIGAN® 0.03% UD from previously prescribed topical IOP-lowering therapy due to medical reasons. Complete data were available for 1,543 patients. AEs were experienced by 5.7% of patients, and there were no serious AEs reported. The most common AEs were eye irritation (1.7%) and hyperemia (1.4%).2
*p<0.0001.
IOP, intraocular pressure; OHT, ocular hypertension; POAG, primary open-angle glaucoma; SD, standard deviation; UD, unit dose.
Real-world evidence is collected outside of controlled clinical trials and has inherent limitations including a lesser ability to control for confounding factors.
LUMIGAN® 0.3 mg/mL unit dose is indicated for the reduction of elevated intraocular pressure in chronic open-angle glaucoma and ocular hypertension in adults (as monotherapy or as adjunctive therapy to beta-blockers).1
In a 3 month clinical study, approximately 29% of patients treated with LUMIGAN® 0.3 mg/mL unit dose experienced adverse reactions. The most frequently reported adverse reactions were conjuctival hyperemia (mostly trace to mild and of a non-inflammatory nature) occurring in 24% of patients, and eye pruritis occurring in 4% of patients. Approximately 0.7% of patients in the LUMIGAN® 0.3 mg/mL single-dose group discontinued due to any adverse event in the 3 month study. The following adverse reactions were reported during clinical trials with LUMIGAN® 0.3 mg/mL single-dose or in the post-marketing period. Most were ocular, mild and none was serious.1
Please refer to LUMIGAN® 0.03% UD Summary of Product Characteristics for further information on adverse events.


